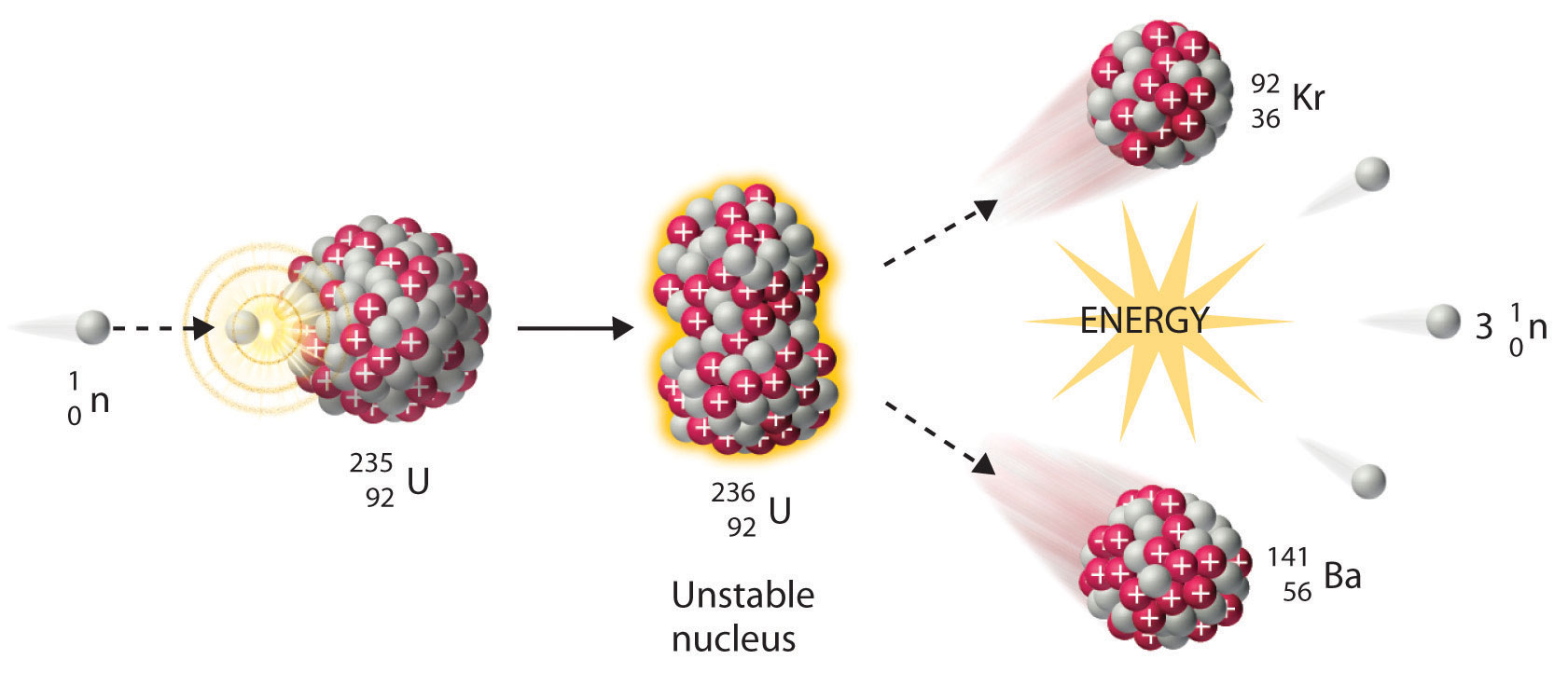
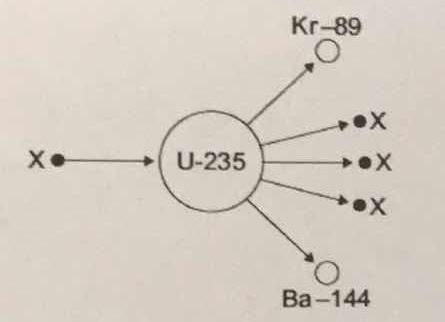
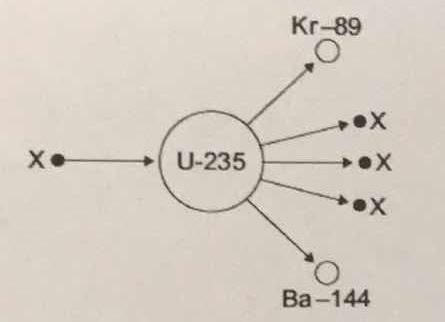
The diagram shows an atom of uranium-235 being split into several pieces. What is the name the process shown in the diagram? What is the name of the particles labelled X?
1 Answer
Here's what I got.
Explanation:
Let's start by identifying the particles labeled
As you know, nuclear reactions are characterized by the fact that
- mass is conserved, i.e. the overall mass number of the ractants must be equal to the overall mass number of the products
- charge is conserved, i.e. the overall atomic number of the reactants must be equal to the overall atomic number of the products
Now, notice that you have
Grab a periodic table and look for the atomic numbers of uranium,
#"For U: " -> "atomic number"= 92#
#"For Kr: " -> "atomic number" = 36#
#"For Ba: " -> "atomic number" =56#
If you take
#""_color(darkgreen)(Z)^color(blue)(A)"X" + ""_color(darkgreen)(color(white)(1)92)^color(blue)(235)"U" -> ""_color(darkgreen)(36)^color(blue)(89)"Kr" + ""_color(darkgreen)(color(white)(a)56)^color(blue)(144)"Ba" + 3""_color(darkgreen)(Z)^color(blue)(A)"X"#
Now, you know that mass and charge must be conserved, which means that you can write
#color(darkgreen)(Z + 92 = 36 + 56 + 3Z)-># conservation of charge
This will get you
#92 = 92 + 2Z implies Z = 0#
You can also write
#color(blue)(A + 235 = 89 + 144 + 3A) -># conservation of mass
This will get you
#2 = 2A implies A = 1#
Therefore, you can say that the particle labeled
#""_Z^A"X" = ""_0^1"n"#
The balanced nuclear equation will look like this
#""_ 0^1"n" + ""_ (color(white)(1)92)^235"U" -> ""_ 36^89"Kr" + ""_(color(white)(a)56)^144"Ba" + 3""_0^1"n"#
This nuclear equation describes the nuclear fission of uranium-235.