The specific heat of silver is 0.24 J/g°C. How many joules of energy are needed to warm 7.37 g of silver from 25.0°C to 27.5°C?
1 Answer
Jan 16, 2017
The amount of energy required is
Explanation:
The equation to use for this problem is written below. We need to determine which variables are known, and which is unknown. Then substitute the known values into the equation and solve.
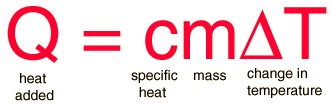
Known
Unknown:
Solution

