How do you perform free radical halogenation?
1 Answer
See below.
Explanation:
When treated with bromine or chlorine, a hydrocarbon can be made into an alkyl halide. Bromine
There are four general steps recognized in a free radical halogenation reaction: initiation, propagation (two steps), and termination. I will use an example to illustrate these steps.
Let's look at the free radical halogenation of butane:

Note that the hydrogens on butane are not written out but are implied. Similarly, lone pairs of electrons on bromine are not shown in the product, but do exist.
The conditions for the reaction include diatomic bromine and heat or UV light, symbolized by
The first step is called the initiation step. In this step, the bromine molecule undergoes homolytic cleavage to form two identical bromine radicals.
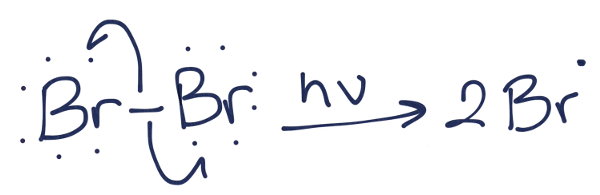
The chain reaction process is now initiated. Next is the first propagation step. In the first propagation step, a bromine radical will abstract a proton from butane to form

Any of the above hydrogens may be abstracted from butane, as they are equivalent.

Note again that there is nothing special about the particular hydrogen I have shown being abstracted by the bromine radical. Also note the use of the single-headed arrow to show the movement of single electrons, whereas a double-headed arrow indicates the movement of an electron pair.
We get:

Bromine now has a full octet and is otherwise satisfied. However, we have now generated another radical. Now our butane radical will abstract a bromine from another

Giving:

Keep in mind that there are many molecules of bromine and butane in the reaction mixture, not just these few! This means that this process will continue until there is nothing left to react. Eventually, the reaction will stop, and this is brought about by the termination step. There are several possibilities for how this occurs. For example, a bromine radical could collide with the wall of the container and be neutralized (not in the acid-base sense). Other options include the reaction of two bromine or two butane radicals. You could also have a bromine and butane radical react. In any of these cases, a free radical is not generated in the products, so the chain reaction is not continued.
Note that the product in which the bromine is bonded to a primary carbon is possible, it's just thermodynamically unfavorable, so we would not expect to see it in large amounts at all. In reality, you might have something like this:
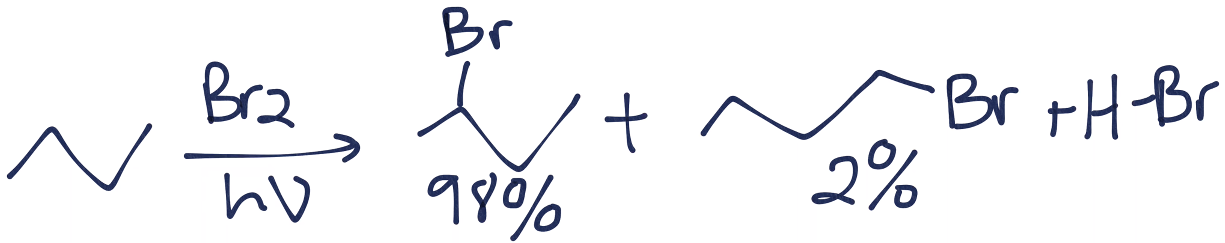
Hope that helps!

