Is water freezing to ice an exothermic or endothermic process?
1 Answer
Jan 29, 2017
Well it's a bond-making process........
Explanation:
And bond-forming processes are exothermic.
On the other hand bond-breaking processes are endothermic.
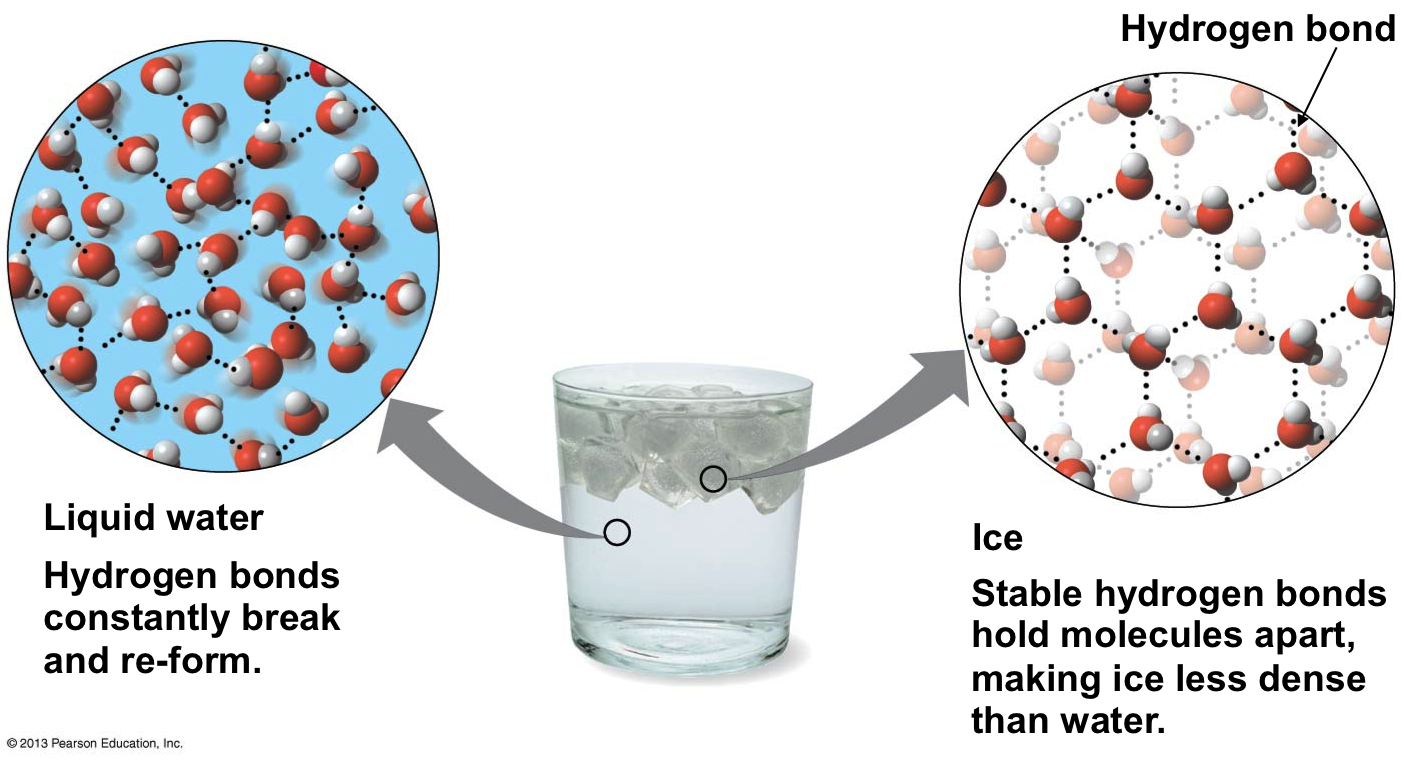
The formation of water-water bonds in a definite array gives rise to the unusual density of ice compared to water. Ice-cubes and ice-bergs float. What does this tell you regarding density?

