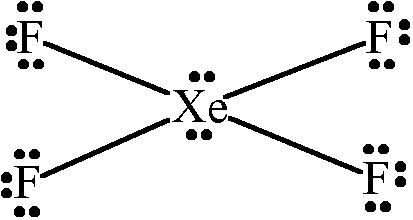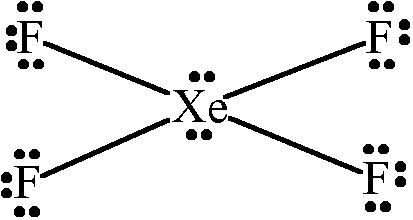London dispersion forces.
Xenon tetrafluoride is #"XeF"_4#, a covalent compound with a square planar geometry. When you draw out the Lewis structure using the electron-counting method:
- #"Xe"# #=># #"8 valence"#
- #4xx"F"# #=># #4xx"7 valence"#
With #36# valence electrons, and fluorine tending to make single bonds, we have #bb8# bonding valence electrons surrounding #"Xe"# for the four single bonds total, and the #bb(4xx6)# nonbonding valence electrons surrounding the fluorine atoms total.
That leaves #4# to be distributed onto xenon, which symmetrically distribute themselves to minimize repulsions.
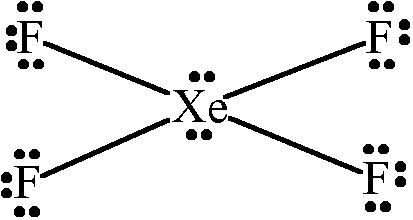
Since #"XeF"_4# is symmetrical, any polarities in its bonds cancel out, leaving it nonpolar. Therefore, it has london dispersion, and that's pretty much it.