How many electrons can go into the third quantum level?
1 Answer
Explanation:
A quick way to tackle this question is by using the equation
#color(blue)(ul(color(black)("no. of e"^(-) = 2n^2)))#
Here
#n# is the principal quantum number
In your case, you will have
#color(darkgreen)(ul(color(black)("no. of e"^(-) = 2 * 3^2 = "18 e"^(-))))#
Now, let's double-check this result by counting the orbitals that are present in the third energy shell.
You know that we can use a total of four quantum numbers to describe the location and spin of an electron in an atom.
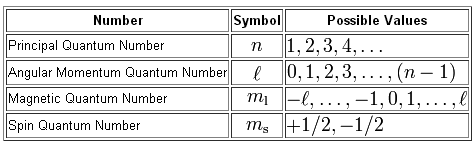
To find the number of electrons that can be located in the third energy shell, which is the energy level that corresponds to
For starters, we know that
#n = 3 implies l = {0, 1, 2}#
This means that the third energy shell holds
Now look at the values that correspond to the magnetic quantum number,
#ul(l = 0 implies m_l = 0) -># the#l=0# subshell can only hold#1# orbital
#ul(l=1 implies m_l = {-1, 0 ,1}) -># the#l=1# subshell can hold#3# orbitals
#ul(l=2 implies m_l = {-2, -1, 0 ,1, 2}) -># the#l=2# subshell can hold#5# orbitals
Therefore, you can say that the third energy shell holds a total of
#1 + 3 + 5 = "9 orbitals"#
According to the Pauli Exclusion Principle, each orbital can hold a maximum of
#color(darkgreen)(ul(color(black)("no. of e"^(-) = 2 * 9 = "18 e"^(-))))#

