What factors increase the solubility of gases?
1 Answer
Feb 21, 2017
Higher atmospheric pressure and lower temperature.
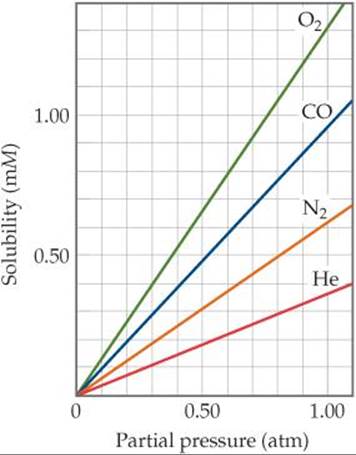
SOLUBILITY OF GASES WITH ATMOSPHERIC PRESSURE
As the atmospheric pressure increases, the gas particles at the liquid's surface are being pushed back into solution more.
In accordance with Henry's law for gaseous substance
#s_i = k_H P_i# ,
where the Henry's law constant
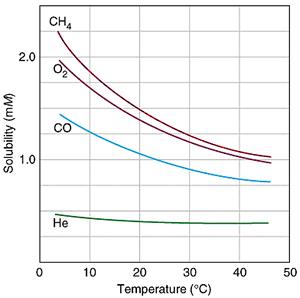
SOLUBILITY OF GASES WITH TEMPERATURE
As you decrease the temperature, the gas moves more slowly, and is thus less prone to escaping the solution. Therefore, its solubility is higher at lower temperatures.