Well, transition metals have #d# orbitals, which match up well in terms of the symmetry, when it comes to interactions with five ring atoms at once.
Basically, only transition metals, with #d# orbitals, are capable of making these bonds, since similar energies, matching symmetry, and optimal overlap are the key criteria to making a chemical bond, and the symmetry requirement cannot be met by #p# orbitals.
It would be literally impossible for, say, carbon, to make 10 interactions. Even in inorganic compounds, the max I have seen carbon make is 6 interactions. Here is an example of carbon making 5 or 6 interactions, to show that I am not kidding:
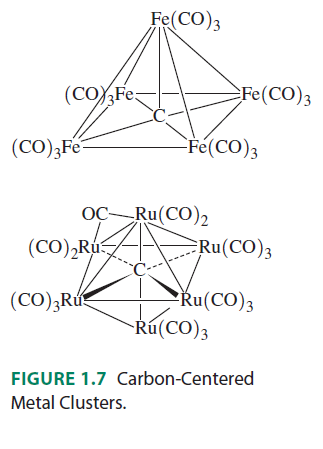
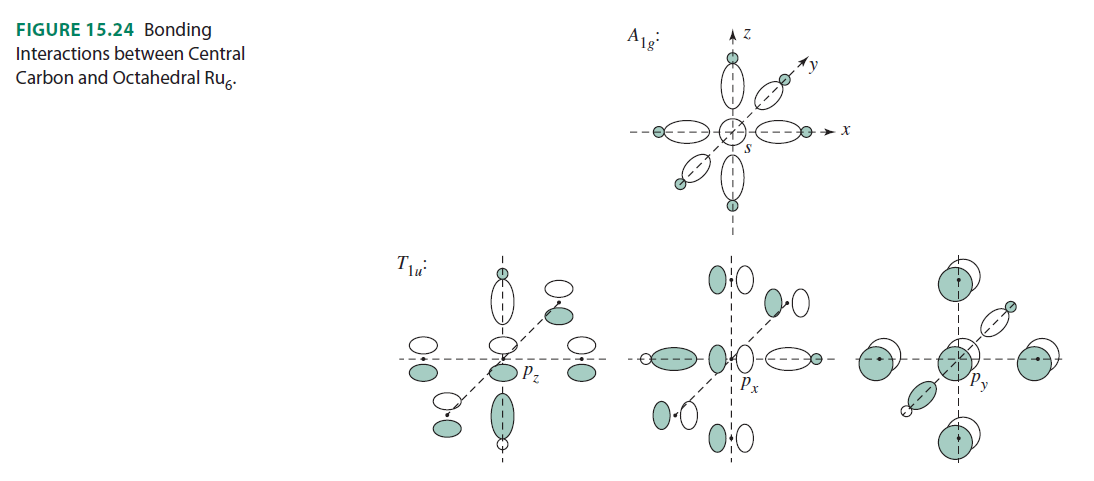
Here are the orbital interactions for the octahedral carbon-centered ruthenium complex (which are all surprisingly just regular #sigma# or #pi# interactions between #ns# and #np# orbitals):
Despite that, it is much more feasible for a transition metal, particularly one with large #d# orbitals, to be at the center of a sandwich compound.
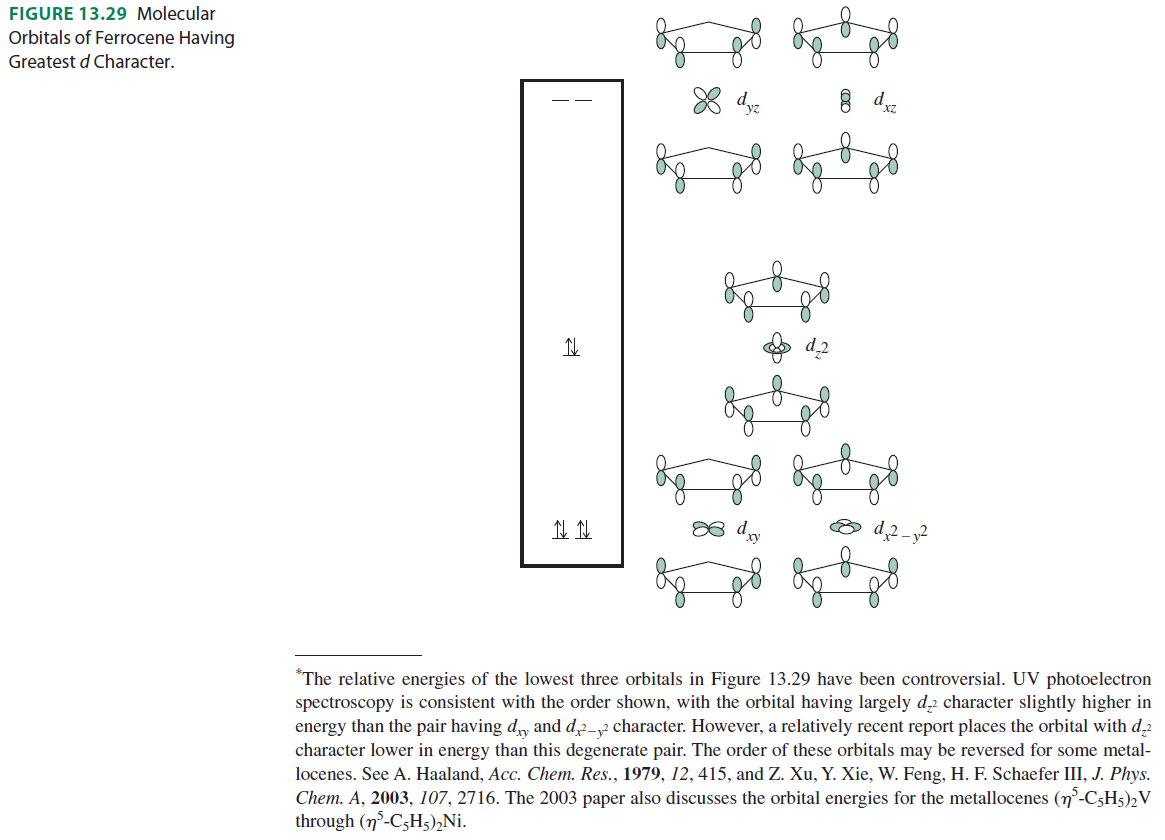
The following are the molecular orbitals of ferrocene with the greatest amount of #d# character:
By inspection of the following character table for #D_(5h)# symmetry, which is the point group of the rings on ferrocene, if you look on the linear and quadratic coordinates columns, those indicate which orbitals pertain to the symmetries of the above molecular orbitals of the two cyclopentadienes as a group.
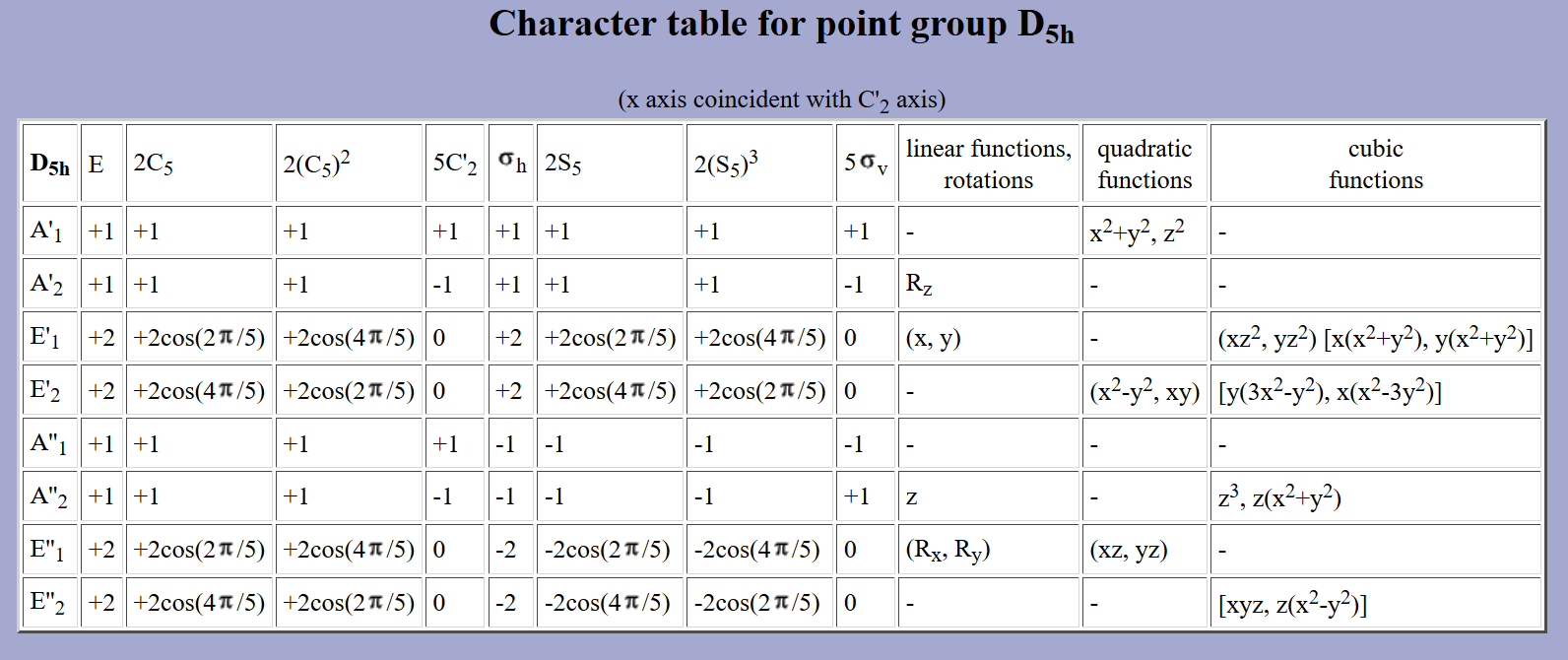
The above orbitals correspond to the irreducible representations called #E_1''#, #A_1'#, and #E_2'#, respectively, since they interact with the [#d_(xz)# and #d_(yz)#], the [#d_(z^2)#], and the [#d_(x^2 - y^2)# and #d_(xy)#] orbitals.
You can see that no #bbx#, #bby#, or #bbz# coordinates pertain to those in the character table (that is, for #p# orbitals; they pertain to #A_2''# and #E_1'#... not matching symmetry!), only #xz#, #yz#, #z^2#, #xy#, and #x^2 - y^2# (all for #d# orbitals).
What this tells us is that in terms of group theory and symmetry, the #bbp# orbitals on non-metals would not be compatible with the orbitals on the two cyclopentadienes as a group, whereas the #bbd# orbitals on transition metals would.
Therefore, carbon, at least, cannot be in the center of two cyclopentadienyl rings, whereas atoms like chromium and iron are just fine with making these pentahapto bonds.

