Are atoms with an atomic number over 83 stable or unstable?
1 Answer
Mar 31, 2017
Unstable
Explanation:
I think you are talking about the different types of decays.
(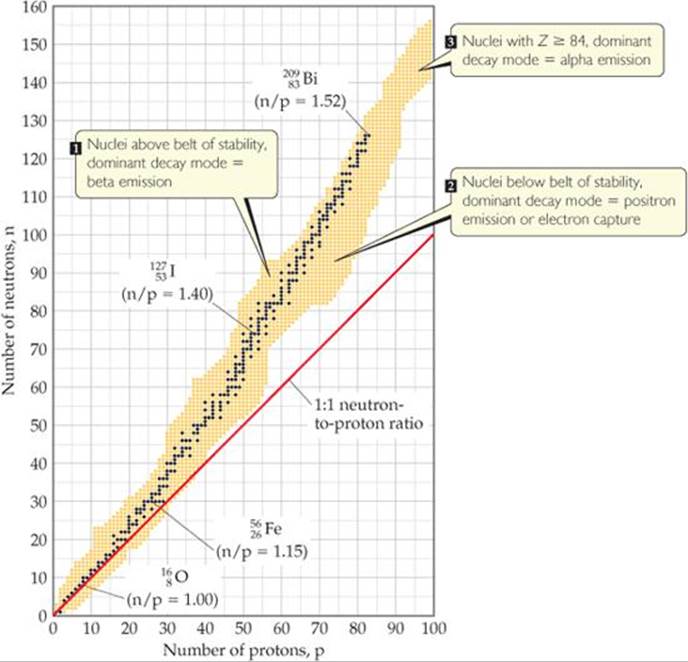 )
)
The dark blue spots depict the belt of stability. An atom with more than 83 protons undergoes alpha decay. For example uraniumhas 92 protons and thus undergoes alpha decay. If an element undergoes any type of decay including positron emission, beta emission or alpha decay the element is unstable. therefore all atoms which have protons more than 83 protons are unstable.

