Alpha Decay
Key Questions
-
An alpha particles is positively charged because it is essentially the nucleus of a Helium-4 atom.
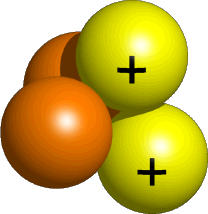
A Helium-4 nucleus is composed of two protons, which are positively charged particles, and two neutrons, which have no electric charge.
A neutral He atom has a mass of four units (2 protons + 2 neutrons) and a net charge of zero because it has two electrons that balance the protons' positive charge; since an
α-particle only has the protons and the neutrons, its charge will be +2→ +1 from each proton. -
Answer:
A Smoke Detector uses the Alpha Particle Decay.
Explanation:
The best known everyday application of alpha decay is the smoke detector. A smoke detector consists of two metal plates with a small space between them. These plates have wires connected to a battery and current monitor. One of the plates contains a small amount of the radioactive element americium, which gives off alpha particles.
These alpha particles complete an electrical circuit between the two plates, and the monitor is inactive. When smoke passes into the smoke detector, it interrupts the flow of alpha particles and the circuit is broken. This action activates the monitor and turns on an audible and visual signal.
And there are tons of uses of alpha particles. You can see the resources available in the internet.
-
Alpha decay is a nuclear change process which produces an alpha particle. Alpha particles are He atoms which have had their electrons removed giving them a +2 charge.
Alpha decay will cause transmutation to occur - this means that one element will turn into another element as the alpha particles are released.
Important connection to history - Rutherford used a radioactive substance (Polonium) in his gold foil experiment when he discovered the nucleus.
Here is a video which discusses how to write and balance an equation for an alpha decay process.
Noel P.