Question #ef73f
1 Answer
Many of the compounds in petrol have only weak intermolecular forces, so they have relatively high vapour pressures.
Explanation:
Petrol is a mixture of many compounds. It can contain 15 % to 60 % alkanes with four to twelve carbon atoms.
The alkanes with fewer than eight carbons all boil below 100 °C, so they have relatively high vapour pressures.
This indicates that there are only weak attractive forces in these compounds.
The
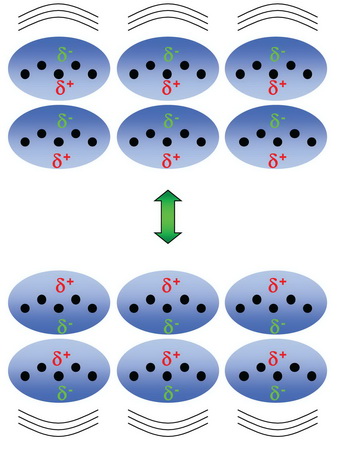
Thus, the molecules need little energy to escape from the surface of the liquid.
If the container is open, you will soon smell the vapours as they escape from the surface of the petrol and move into the air.

