At what temperature can we dissolve equal amounts of ammonium chloride and hydrogen chloride in the same amount of water?
1 Answer
Apr 18, 2017
Equal amounts can be dissolved at 58 °C.
Explanation:
I presume that you are asked to interpret a solubility curve like the one below.
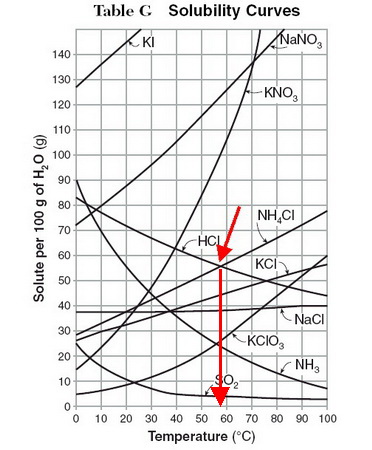
(Adapted from KentChemistry HOME)
Find the curves for
Then, drop a line down to the temperature axis and note the temperature.
In this diagram, it looks like both compounds have the same solubility (about 56 g/100 g of water) at 58 °C.

