Will sodium fluoride and calcium nitrate react to produce a precipitate?
1 Answer
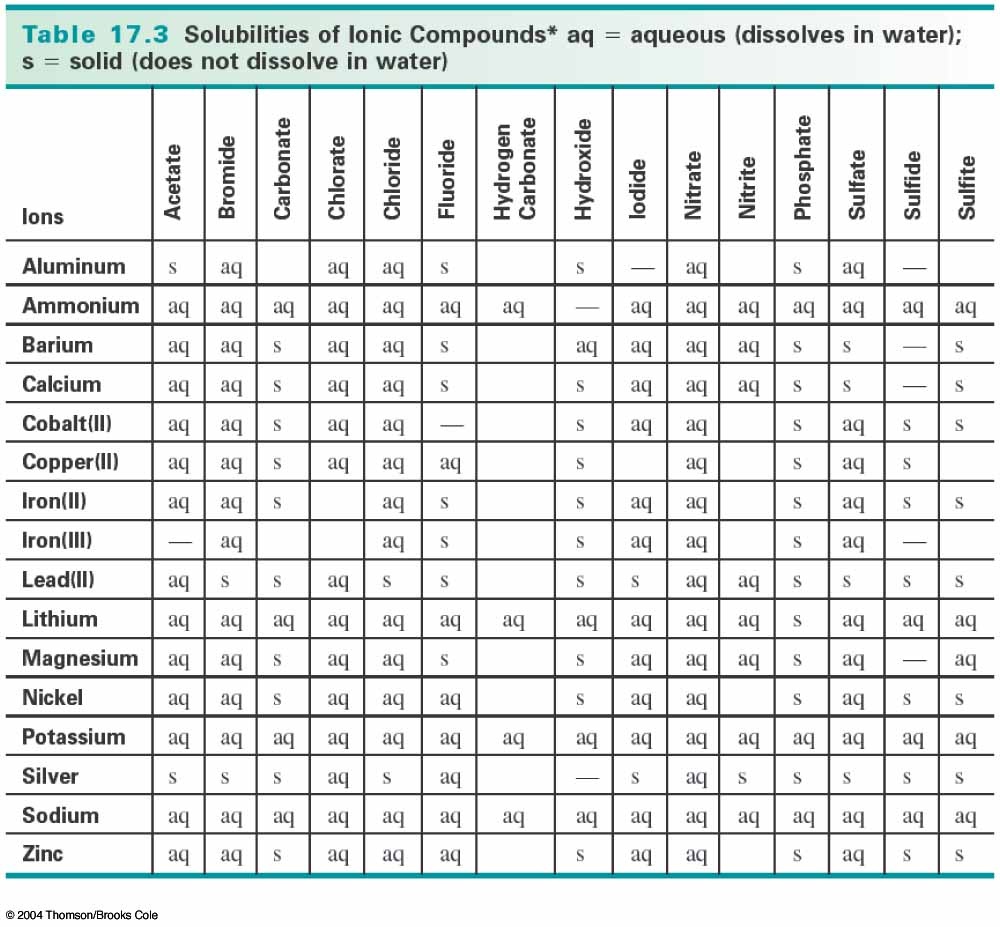
There is a precipitate, which is calcium fluoride,
Explanation:
Balanced Equation
As you can see, a precipitate (solid) does form, and it is calcium fluoride
This is an example of of a double replacement (double displacement or metathesis) reaction. Evidence of a double replacement reaction include a precipitate, or an insoluble gas must bubble out of solution, or water must be a product (neutralization reaction). The generic equation that represents a double replacement reaction is:
where
There are solubility rules that can help you determine whether a precipitate will form, and what it is. As you can see, fluoride and calcium ions form a solid, as designated by an "s".