Question #30ceb
1 Answer
The term 'Cathode' is the site of a reduction reaction in an electrochemical process and a cation is a positively charged particle. This couples with the term 'Anode' which is the site of an oxidation reaction in the electrochemical process and an anion is a negatively charged particle.
Explanation:
WARNING! Long Answer => b/c too many students are defining the Cathode and Anode in terms of charge, which is INCORRECT.
Cathodes and Anodes in electrochemical cells are defined in terms of the chemistry that occurs at the given electrode. Cathodes are the site of the reduction process and Anodes are the sites of the oxidation process.
To be clear, Electrochemistry is divided into two separate topics depending on the spontaneity of then redox process. One, is Electrolysis and is not a spontaneous process and requires the input of energy to drive the electrochemical process. The other process is the Galvanic process which is a spontaneous and generates electrical energy from chemical process.
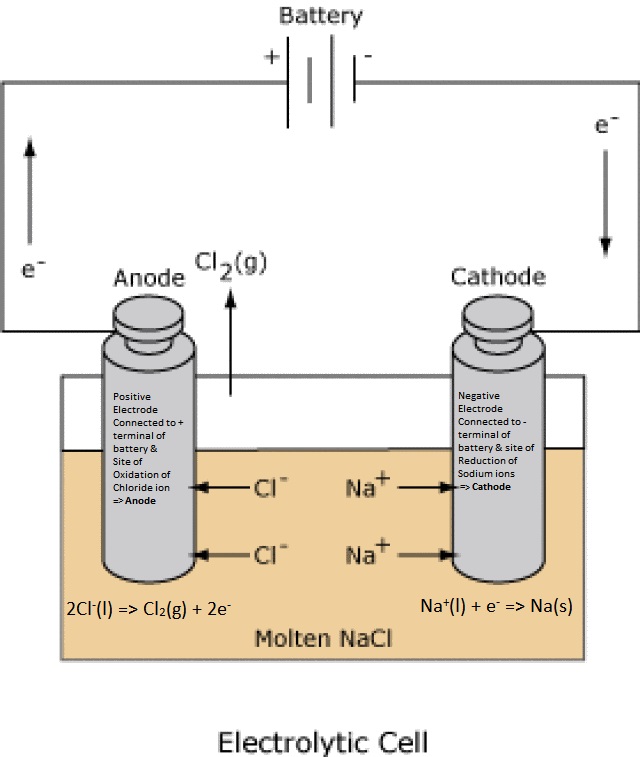
In the Electrolysis Cell, electrodes are connected to a power/energy source (battery) because the chemistry is a non-spontaneous process. Each terminal assumes the charge of the terminal to which they are connected. The terminal that is positive attracts anions
(- charged particles) which deposit electrons (i.e, anions lose electrons) into that electrode by an oxidation rxn (=> Anode).
The electrons then flow through the battery to the other electrode which is negative and attracting cations that undergo reduction (=> Cathode). Example (melt electrolysis of NaCl): The electrode connected to the positive terminal of the battery attracts chloride ions that undergo oxidation.
The electrode connected to the negative terminal attracts positive ions that undergo reduction rxn (=> Cathode) by drawing electrons out of that electrode.
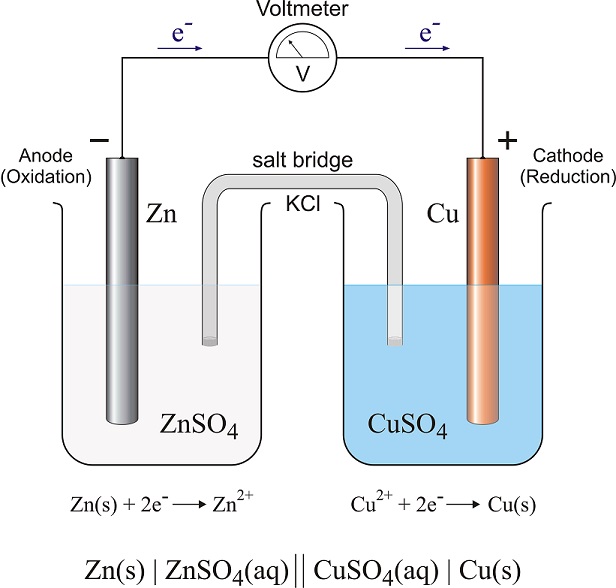
In the Voltaic Cell (Galvanic Process) the electrochemical process is a spontaneous oxidation-reduction reaction. The selective coupling of one substance that will undergo oxidation relative to another substance that will undergo reduction produces a spontaneous flow of electrons.
If the oxidation rxn process is separated from the reduction process a sustained charge flow occurs. This is generally referred to as a 'Controlled Galvanic Process' and is the technical foundation for the chemical battery.
In this cell, the substance undergoing the oxidation reaction (=> Anode) delivers cations into an electrolytic solution leaving behind an excess of electrons and induces a negative charge on that electrode.
The substance undergoing the reduction reaction (=> Cathode) undergoes a gain of electrons leaving that electrode deficient in electrons and induces a positive charge. To compare the charges of the electrolytic cell to the galvanic cell finds them to be opposite for the Cathode and Anode.
In the electrolytic cell the Anode is positively charged while in the galvanic cell it is negatively charged. The Cathode in the electrolytic cell is negative while the cathode in the galvanic cell is positive.
In summary, for the electrochemical process the electrodes are always defined in terms of the chemistry occurring at a designated electrode; not charge. The charges on the cathode and anode of an electrolytic cell are opposite to the charges on the same named electrodes in a galvanic cell.


