Question #c29e5
1 Answer
May 9, 2017
because 35 °C is above the critical temperature of
Explanation:
The critical temperature is the temperature above which a gas cannot be liquefied by pressure alone.
The critical temperature of
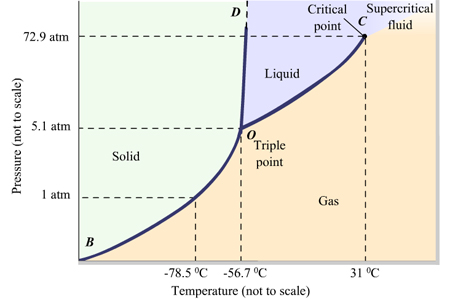
We can see that, if the temperature is 35 °C, an increase in pressure will create only a supercritical fluid, a state in which the gas and liquid phases cannot be distinguished from each other.

