Question #ccc1e
1 Answer
An exploding firework is basically a sequence of chemical reactions happening simultaneously or one after another.
Basically an example of a redox reaction.
Explanation:
A red firework would consist of a mix of oxidizing agent, reducing agent, colouring agent (in this case, the metal salt used would contain Strontium because it produces a bright red colour when ignited).
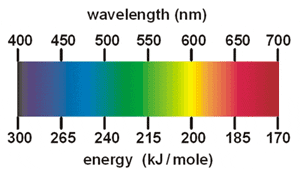
If you want, we can go even further and say that this red colour is produced because the atoms in strontium (Sr) absorb light and emit it at a particular wavelength (652 nm) which is seen as a red colour in the visible part of the spectrum.
On to the oxidation and reduction that is happening in an exploding firework:
The most commonly used oxidizing agents are nitrates, chlorates and perchlorates.
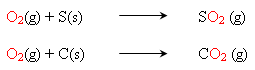
The reducing agents sulphur and carbon combine with the oxygen (from the oxidizing agent) to produce the energy needed for the explosion.
One common oxidizing agent is potassium nitrate, which decomposes to give potassium oxide, nitrogen oxide and oxygen gas.
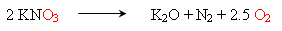
The oxygen releasted by the nitrates (chlorates or perchlorates) combine with the reducing agents sulphur and carbon to produce sulphur dioxide and carbon dioxide respectively.