Question #5aa17
2 Answers
The chemical reaction is based from the number of moles of each species participating in the reaction and not by its volume.
Explanation:
This is how it works:
First, this is the reaction for the formation of water:
All chemical reactions are based on the number of moles (see those coefficients?) and not in their volume. Meaning 1 mole of
The answer lies in Avogadro's Hypothesis.
Explanation:
Avogadro's Hypothesis states that equal volumes of gases at the same temperature and pressure contain equal numbers of molecules.
The chemical equation for the formation of water is
#"2H"_2"(g)" + "O"_2"(g)" → 2"H"_2"O(g)"#
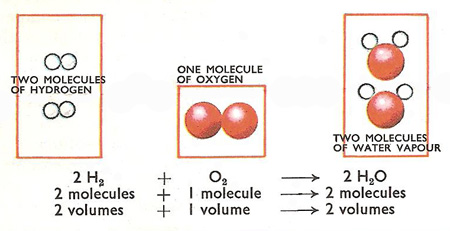
We could also write
#"2 mL + 1 mL → 2 mL"#
or
#"100 mL + 50 mL → 100 mL"#
The volumes will always be in the same