What could cause a gaseous substance to liquify?
1 Answer
Temperatures at or below a critical temperature,
Explanation:
Anytime a substance (solid or gas) is turned into a liquid, the process is called liquefaction. When turning gas into liquid, the distance between gas molecules is large compared to the distance between liquid molecules. So there is a lower density in the gaseous form and a higher density in liquid form. So, when a gas turns into a liquid, it's called condensation.
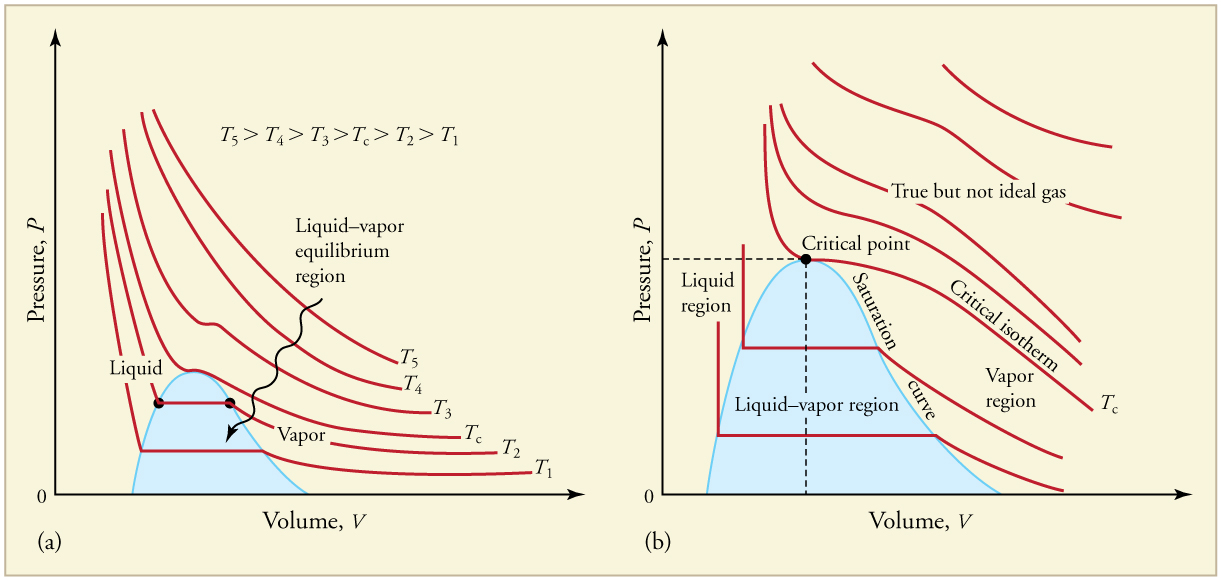
Ideal gases do not get converted into liquid form. So the gas must be a real gas. Three steps are required for liquefaction:
- Start with a gas only
- Vapor and liquid exist in equilibrium (vapor
#rightleftharpoons# liquid) - End with a liquid only
This process requires varying temperature and pressure at the same time. Neither keeping a fixed temperature and only changing pressure, nor keeping a fixed pressure and only changing temperature will work. Instead, there must be some constraints on both temperature and pressure.
If the temperature is too high, the molecules will be moving too quickly to allow the intermolecular forces of attraction to form the required gas
Similarly, low pressures result in greater distances between the molecules, while higher pressures will result in shorter distances. Thus, a low temperature/high pressure combination will be required to liquefy a gas. Note that if the temperature and pressure conditions are such that gas can liquefy, we call the gas vapor.
Using a graph of isotherms for a gas of interest (e.g.,