What is the oxidation state of sulfur in #"S"_4"O"_6^(2-)#?
1 Answer
#-2# for the two sulfur atoms forming the central, disulfide bridge.#+6# for the two sulfur atoms central to each thiosulfate fragment.
If we peer over at Wikipedia, we obtain the Lewis structure...
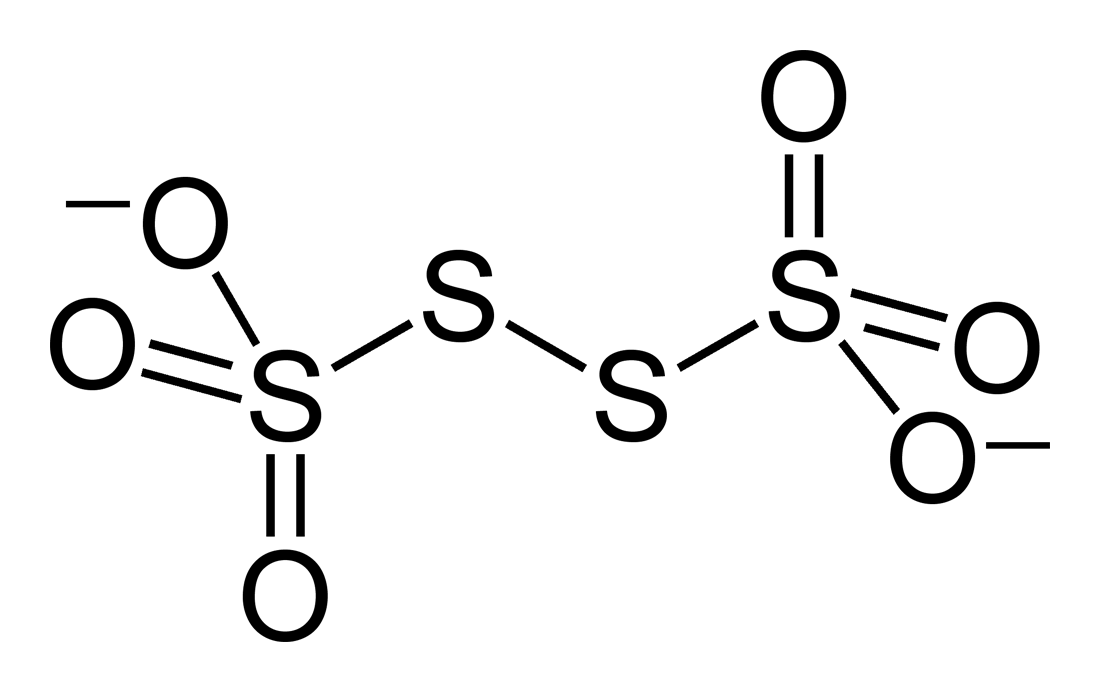
It turns out that this is analogous to a peroxide with sulfur atoms (notice the disulfide bond,
We also know that the two central sulfur atoms have the same oxidation state, just as the two oxygen atoms do in
Consider cleaving the ion into two (identical) thiosulfate,
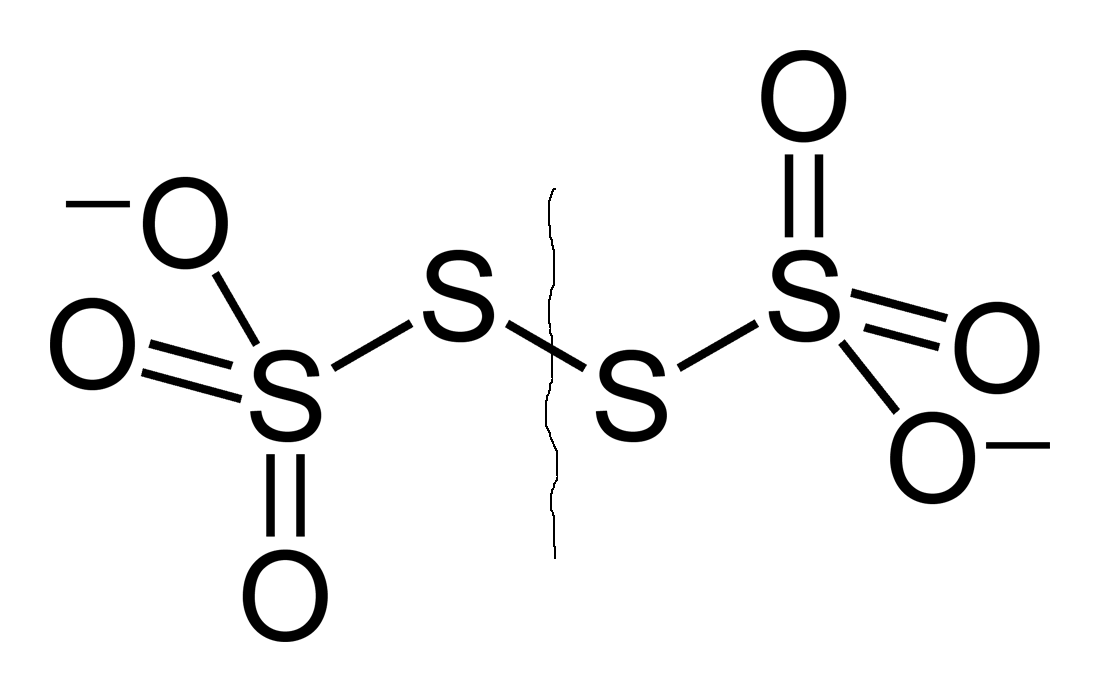
Now we simply examine one of the thiosulfate halves of the original ion.
Due to the two sulfur environments, the outer thiosulfate sulfur has a different oxidation state, so we write the following equation for the oxidation states within thiosulfate:
#stackrel("O atom")overbrace(3(-2)) + stackrel("inner S atom")overbrace(S_i) + stackrel("outer S atom")overbrace(S_o) = -2#
By analogy with sulfate,
Therefore, the inner sulfur oxidation state is:
#S_i = -2 - 3(-2) - S_o#
#= -2 + 6 - (-2)#
#= +6#
Since the tetrathionate ion is symmetric about the cleavage line we just drew, we have found the two sulfur oxidation states:
#color(blue)(-2)# for the two sulfur atoms forming the central, disulfide bridge.#color(blue)(+6)# for the two sulfur atoms central to each thiosulfate fragment.

