How do we determine the covalency of various molecules?
2 Answers
You will have to give some context to your question........
Explanation:
A better term than
Draw the Lewis structure and count the number of shared electron pairs.
Explanation:
Covalency is the number of bonds an atom forms within a molecule.
To determine the covalency, you draw the Lewis structure of the molecule and count the number of shared electron pairs.
Here are some examples.
Covalency = 1


Hydrogen and chlorine can each form one bond. can form one bond.
Covalency = 2

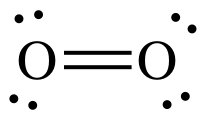
Oxygen atoms can form two bonds.
Covalency = 3
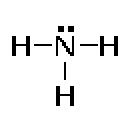

A nitrogen atom can form three bonds.
Covalency = 4


A carbon atom can form 4 bonds.
Covalency >4

Many atoms with atomic number greater than 14 can form 5 or 6 bonds.


