How does an atom with too many neutrons relative to protons undergo radioactive decay?
1 Answer
Explanation:
If a nucleus is unstable due to too many neutrons, it will undergo Beta decay - this means they become stable by emitting a beta particle.
Beta particles are essentially fast moving electrons. To make the atom stable again, one of the neutrons changes into a proton. Because a proton is positive whilst a neutron is neutral, this means that somehow we need to find a way to account for the lost negative charge - so as well as a proton, the neutron also produces a high energy electron (think of the negativity of the electron balancing out the positivity of the proton to make a neutron neutral). This causes the atomic mass to remain unchanged (electrons have such a small mass that there is no point counting it for this ) but the atomic number increases by one because the atom has essentially gained a proton and lost a neutron.
This shifts the ratio back because there are fewer neutrons AND more protons.
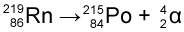
An example is Carbon-14 (atomic number 6) releasing a beta particle to become Nitrogen-14 (atomic number 7).
Note that the atomic number (number of protons) has increased by one, and the mass has stayed the same.
Beta particles are between alpha particles and the third type of ionising radiation - gamma rays - in terms of how strongly ionising they are. They are also in the middle for how penetrating they are and will pass through paper but be stopped by aluminium foil (a few millimetres thick). It has a range in air of around 15cm.
I hope this has helped! Let me know if I can do anything else, as this is one of my favourite areas of physics and so I would love to talk about it:)
