Question #fe00e
1 Answer
When an element is reduced its oxidation state becomes more negative due to gain of electrons, and when an element is oxidized its oxidation state becomes more positive due to loss of electrons.
Explanation:
The process of oxidation and reduction is based upon the gain and loss of equal numbers of electrons in the reactive process. By definition, oxidation is the loss of electrons (=> more positive oxdn state) and reduction is the gain of an equal number of electrons (=> giving a more negative oxdn state).
Example:
The function of a simple Voltaic Cell ( Chemical Battery) is based upon the flow of electrons in the controlled coupling of oxidation-reduction reactions. For a Zinc/Copper Voltaic Cell the following reaction process occurs:
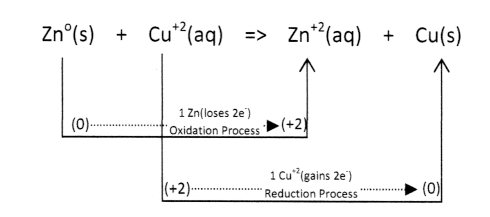
Required:
=> electrons lost (oxidation) = electrons gained (reduction)

