Question #6d808
1 Answer
No, a reaction will not occur as-is. The activation energy must be surpassed, but it was not;
Activation energy is defined as the energy threshold that must be overcome in order for a reaction to occur. Consider the general reaction
#X + Y -> Z#
with the reaction coordinate diagram:
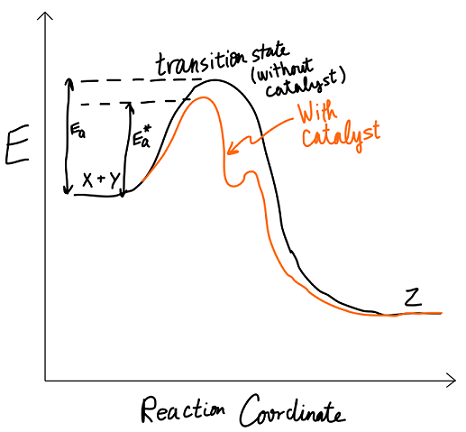
We stated that
EASIEST FIX
But if we add a catalyst, like an enzyme, then a different reaction mechanism will occur, with a lower first
If we have that
CREATIVE FIX
In the easier fix, we lowered the barrier.
This other option is not obvious, but we could also raise the energy of the reactants (if we can't lower the barrier, we could raise ourselves to be closer to the top).
So, we could pump in some heat before the reaction occurs, and then place the reactants in the vessel. The reactants then take in that heat and start at a higher energy.
The resultant reaction should be more exothermic if it was exothermic previously, or less endothermic if it was endothermic previously.

