Why is the #"FPF"# bond angle larger than the #"BrPBr"# angle in #"PF"_3# and #"PBr"_3# respectively?
1 Answer
It's not... The
http://cccbdb.nist.gov/expangle1.asp
In fact, if you look closely here at the electron density maps, you can kind of see the increase in bond angle from
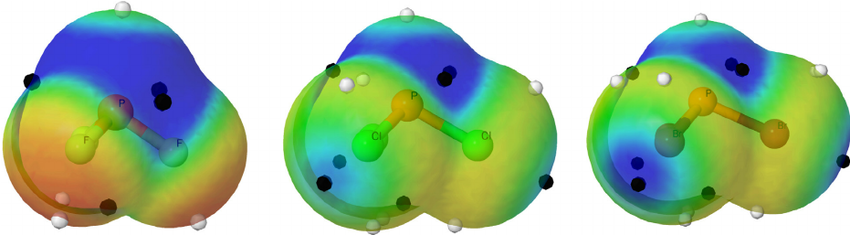
We actually have two factors here:
- size of the terminal atoms (and thus the
#"XPX"# bond lengths) - electronegativity of the terminal atoms
SIZE FACTOR
Since
The atomic radius difference also allows each fluorine atom to be closer together than the bromine atoms can be, without experiencing as much electron repulsions from each terminal atom's lone pairs.
(Or, we could even say that the repulsive torque from the bonding-electron pairs is less on the
ELECTRONEGATIVITY FACTOR
Furthermore, the electron density is more localized onto the fluorine atoms due to their higher electronegativity than that of bromine atoms. Electron density localized in the bonds is on average farther away when we move away from the peak of the molecule (the lone pair).
These together facilitate the smaller bond angle of

