Question #79d88
1 Answer
Explanation:
The total number of valence electrons in a molecule of acetaldehyde,
A molecule of acetaldehyde contains
- two atoms of carbon,
#2 xx "C"# - four atoms of hydrogen,
#4 xx "H"# - one atom of oxygen,
#1 xx "O"#
Now, you should know that you have
#"For C: 4 valence electrons"# #"For H: 1 valence electron"# #"For O: 6 valence electrons"#
This means that the total number of valence electrons present in a molecule of acetaldehyde is equal to
#"no. of e"^(-) = overbrace(2 xx "4 e"^(-))^(color(blue)("from 2 atoms of C")) + overbrace(4 xx "1 e"^(-))^(color(darkorange)("from 4 atoms of H")) + overbrace(1 xx "6 e"^(-))^(color(purple)("from 1 atom of O"))#
#"no. of e"^(-) = "18 e"^(-)#
You can double-check the calculations by looking at the Lewis structure of acetaldehyde, or ethanal.
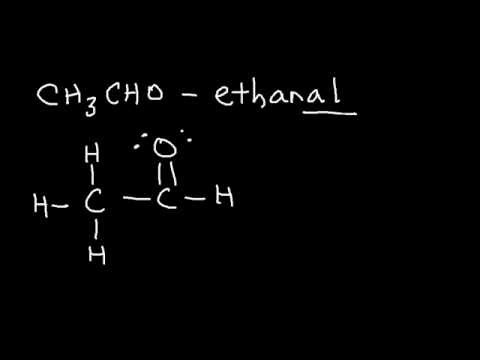
You know that each single bond is made up of
As you can see, you have
#5 xx "2 e"^(-) + 1 xx "4 e"^(-) = "14 e"^(-)#
The two lone pairs of electrons present on the oxygen atom bring the total number of valence electrons to

