Question #c7688
1 Answer
Here's what I got.
Explanation:
The trick here is to realize that peroxydisulfuric acid contains the peroxydisulfate anion,
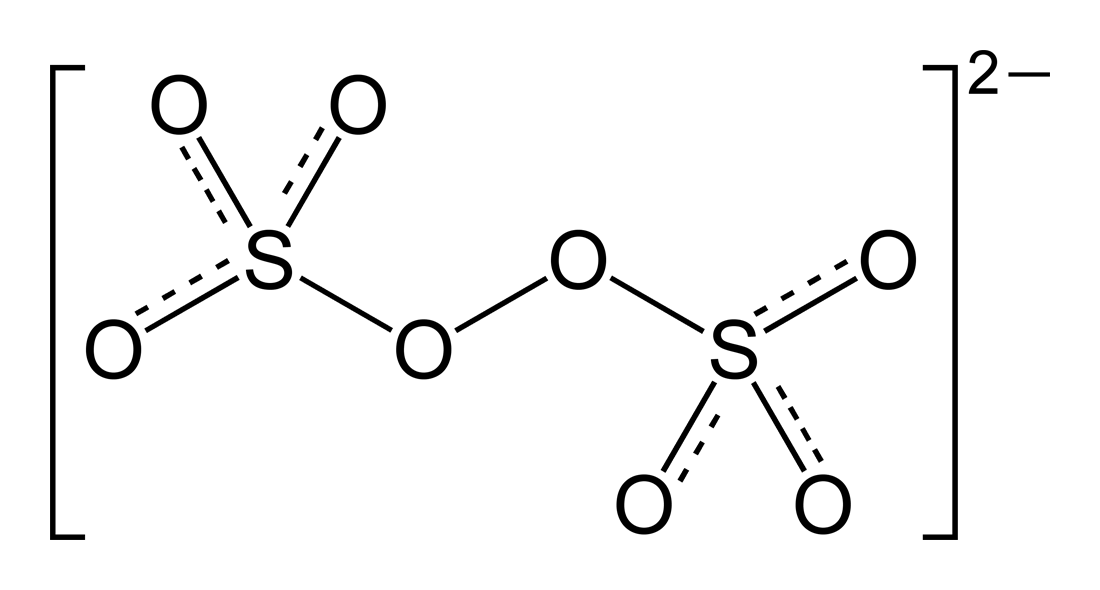
Notice that two oxygen atoms are bonded via a single bond--this is known as a peroxide linkage. This will influence the average oxidation state of oxygen in this anion.
More specifically, the two oxygen atoms that are bonded to each other will have a
The other six oxygen atoms will have a
#(6 xx color(blue)(-2) + 2 xx color(blue)(-1))/8 = -7/4#
This means that instead of using two different oxidation states for the oxygen atoms, you can use an average oxidation state of
This implies that you have--keep in mind that hydrogen has a
#stackrel(color(blue)(+1))("H")_ 2 stackrel(color(blue)(?))("S") _ 2 stackrel(color(blue)(-7/4))("O")_ 8#
Now, peroxydisulfuric acid is a neutral compound, which implies that the sum of the oxidation states of all the atoms that make up a molecule of peroxydisulfuric acid must be equal to
This means that you have
#overbrace(2 xx color(blue)((+1)))^(color(red)("2 atoms of H")) + overbrace( 2 xx ?)^(color(red)("2 atoms of S")) + overbrace(8 xx color(blue)((-7/4)))^(color(red)("8 atoms of O")) = 0#
Solve to find the oxidation state of sulfur
#2 * 2 * ? - 14 = 0#
#2 * ? = 12 implies ? = +6#
Therefore, you can say that sulfur has a
#stackrel(color(blue)(+1))("H")_ 2 stackrel(color(blue)(+6))("S")_ 2stackrel(color(blue)(-7/4))("O")_ 8#
Now, here's an example of how NOT to answer this question.
Your ultimate goal here is to figure out the oxidation state of sulfur in peroxydisulfuric acid,
More specifically, hydrogen has a
This means that you have
#stackrel(color(blue)(+1))("H")_ 2 stackrel(color(blue)(?))("S")_ 2stackrel(color(blue)(-2))("O")_ 8#
Now, peroxydisulfuric acid is a neutral compound, which implies that the sum of the oxidation states of all the atoms that make up a molecule of peroxydisulfuric acid must be equal to
This means that you have
#overbrace(2 xx color(blue)((+1)))^(color(red)("2 atoms of H")) + overbrace( 2 xx ?)^(color(red)("2 atoms of S")) + overbrace(8 xx color(blue)((-2)))^(color(red)("8 atoms of O")) = 0#
Solve to find the oxidation state of sulfur
#2 + 2 * ? - 16 = 0#
#2 * ? = 14 implies ? = 14/2 = 7#
Therefore, sulfur is in a
#stackrel(color(blue)(+1))("H")_ 2 stackrel(color(blue)(+7))("S")_ 2stackrel(color(blue)(-2))("O")_ 8#

