Each of the following reactions shows a solute dissolved in water. Classify each solute as a strong electrolyte, a weak electrolyte, or a nonelectrolyte?
"M"(l)→"M"(aq)
"AC"(aq)⇌"A"^(+)(aq) + "C"^(−)(aq)
"BD"(s)→"B"^(+)(aq) + "D"^(−)(aq)
"PR"(aq) → "P"^(+)(aq) + "R"^(−)(aq)
"N"(s)→"N"(aq)
1 Answer
Here's what I got.
Explanation:
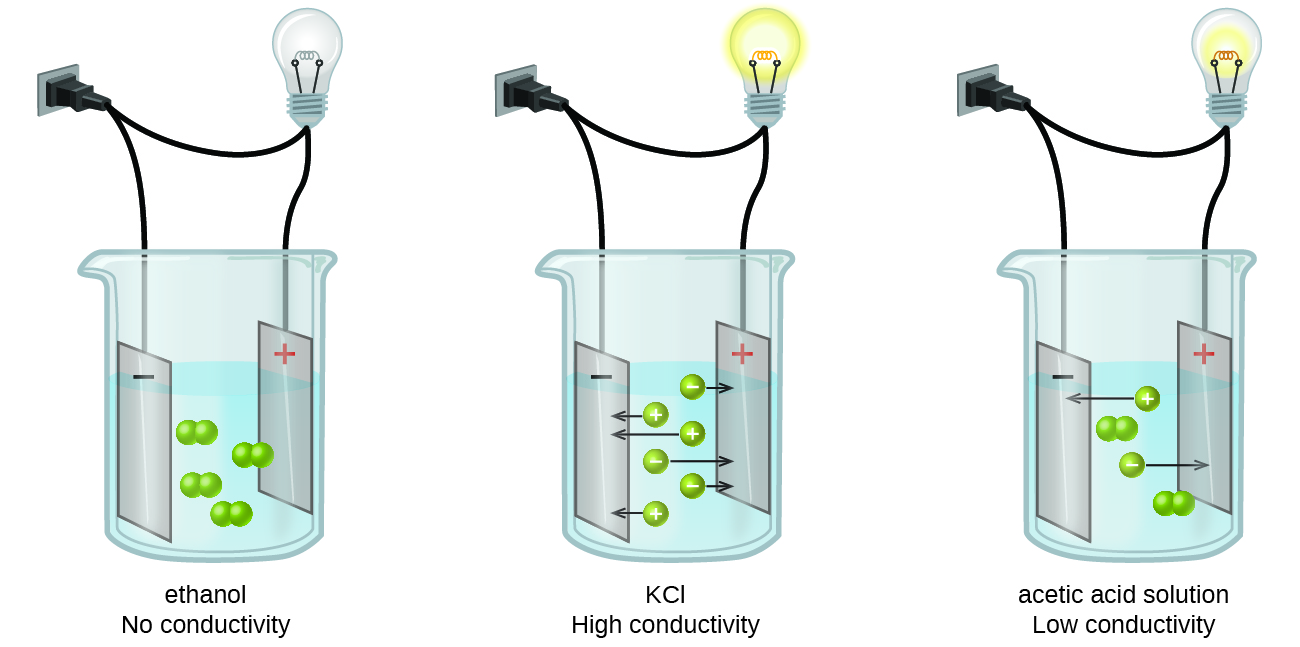
For starters, you should know that an electrolyte is simply a substance that dissolves in water to produce a solution that can conduct electricity.
Simply put, an electrolyte is a substance that produces ions when dissolved in water. Consequently, a non-electrolyte is a substance that does not produce ions when is dissolved in water.
 http://philschatz.com/chemistry-book/contents/m51086.html
http://philschatz.com/chemistry-book/contents/m51086.html
Now, the first thing to look out for here is the equilibrium arrow,
We use this symbol to show that the reaction does not go to completion, which basically means that, at equilibrium, the reaction vessel contains both reactants and products.
In your case, you have
"AC"_ ((aq)) rightleftharpoons "A"_ ((aq))^(+) + "C"_ ((aq))^(-)
The fact that an equilibrium arrow is used tells you that a solution of
In other words,
color(blue)(ul(color(black)("weak electrolyte = partially ionizes to produce ions")))
By comparison, we use a regular reaction arrow,
In your case you have
"BD"_ ((s)) -> "B"_ ((aq))^(+) + "D"_ ((aq))^(-)
This means that when
color(blue)(ul(color(black)("strong electrolyte = completely ionizes to produce ions")))
The same can be said about
"PR"_ ((aq)) -> "P"_ ((aq))^(+) + "R"_ ((aq))^(-)
You can thus say that
Finally, notice that
"M"_ ((l)) -> "N"_ ((aq))
"N"_ ((s)) -> "N"_ ((aq))
This implies that they are non-electrolytes.
color(blue)(ul(color(black)("non-electrolyte = does not produce ions")))

