In standardization, 8.77 mL of #NaOH# neutralized 1.522 g of #KHP#. Given the molar mass of #KHP# is 204.22 g/mol, what is the molarity of the #NaOH# solution?
1 Answer
Jun 13, 2017
Explanation:
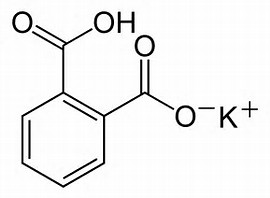
It can be titrated as a base, or here as an acid. It is air stable, non-hygroscopic, and thus useful as a primary standard.
And thus moles of

