Using Ellingham diagram,how to determine that in between C and CO which is better reducing agent?
1 Answer
It all depends on the temperature and what you are trying to reduce.
Explanation:
An Ellingham diagram is a plot of ΔG versus temperature for different reactions. For example,
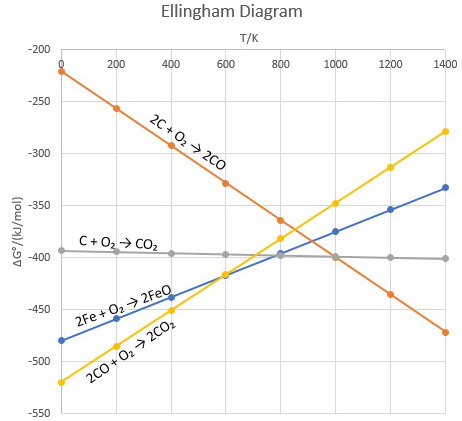
A key point in the graphs is the point where two reaction lines cross.
At this point,
On either side of the crossover point, the reaction represented by the lower line (the one with the more negative value of
Thus, it is possible to predict the temperature above which, say, carbon or carbon monoxide will reduce any metal oxide, for example
Below 600 K, only
Above 800 K, reduction by conversion of coke to carbon dioxide is spontaneous.
Above 900 K, reduction by conversion of coke to carbon monoxide is spontaneous.
Thus, for the reduction of

