How do you write an equation for the beta decay of sodium-24?
1 Answer
Here's how you can do that.
Explanation:
Sodium-24 undergoes beta decay, or, more specifically, beta-minus decay.
During a beta decay, a neutron located in the nucleus of a radioactive nuclide is converted to a proton. At the same time, the nuclide emits an electron, also called a beta particle, and an electron antineutrino,
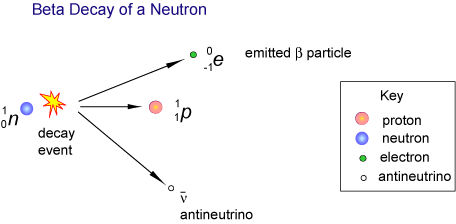
Since a neutron is being converted to a proton, you can say that the atomic number of the nuclide increases by
You will thus have
#""_ 11^24"Na" -> ""_ Z^A"?" + ""_ (-1)^(color(white)(-)0)beta + bar(nu)_"e"#
In any nuclear reaction, charge and mass must be conserved, so you have
#24 = A + 0 -># conservation of massThis will get you
# A = 24 -># the mass number remains unchanged!
#11 = Z + (-1) -># conservation of chargeThis will get you
#Z = 12 -> # he atomic number increases by#1# !
Therefore, you can say that the resulting nuclide will have
This means that you have
#""_ 11^24"Na" -> ""_ 12^24"Mg" + ""_ (-1)^(color(white)(-)0)beta + bar(nu)_"e"#

