How would you define and explain periodic trends?
1 Answer
Here's what I have..
Explanation:
WARNING: LONG ANSWER!
The four primary periodic trends I can think of are trends in
-
atomic radius
-
ionization energy
-
electron affinity/electronegativity
-
metallic character
The atomic radius of an element is, for general purposes, the distance from the center of the atom's nucleus to the outermost space occupied by electrons in the "electron cloud". There are several types of atomic radii, but we need not preoccupy ourselves with that, as they generally all follow the same trends.
Going left to right across a period on the periodic table, the general trend is that the radius decreases with each successive element. Why is this so?
For each successive element you come across, the atomic number, and thus the number of protons and electrons, increases by
#1# . Recall that the positively-charged protons attract the electrons toward the nucleus. The pull the protons exert on the electrons is greater in magnitude than the pull the electrons exert on the protons, so the overall change is that the electrons are pulled in closer, and thus the "size" of the atom has decreased.Going from top to bottom within a group on the periodic table, the general trend is that the radius increases. Why?
Each successive element you come across is in a new period . This means that the outer electrons occupy a different shell that is farther from the nucleus (the period number increases as you go down a group). The size of the atom thus increases as you go down a group.
Here is a visual periodic table representing this:
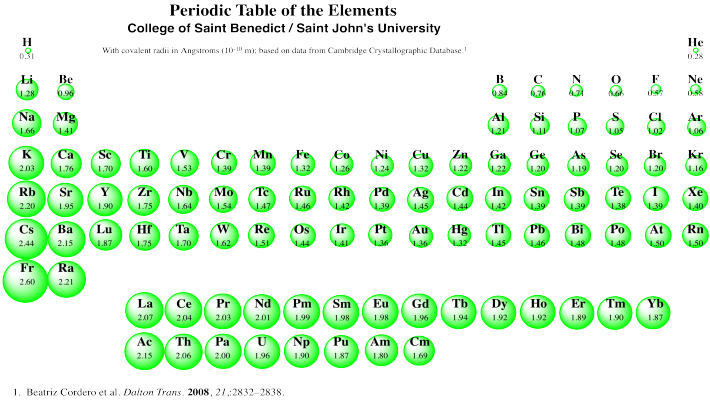
The ionization energy of an element is the amount of energy required to remove the most loosely-bound electron from an isolated ("gaseous") neutral atom.
Going left to right across a period, the general trend is that ionization energy increases. Why?
Going left to right, the number of valence electrons increases by one, and the valence shell thus becomes more and more filled. You may recall that nonmetals (those on the right side with more filled valence shells) tend to gain electrons to fill its valence shell. If they "would rather gain an electron than lose one", the energy required to take away an electron would thus be higher.
For metals, whose valence electrons aren't filled very much, they require much less energy to lose an electron (hey "prefer" to lose electrons to become cations) so therefore ionization energy generally increases from left to right.
Going down a group, the ionization energy generally decreases.
This is due to a property called electron screening. What this basically is, is that the inner electrons act as a "screen" or "shield" that "block" the attraction to the nucleus, and thus the electrons are farther from the nucleus, and are easier to ionize because less energy is required. Going down a group, there are more inner electrons to "screen" the effects of the nucleus, so the ionization energy decreases
The electronegativity of an element is a measure of how likely, when in a bond with another atom, that atom will "pull" the bonding electrons toward itself.
The general trend when going left to right across a period is that the electronegativity increases.
This is due primarily to the fact discussed in the ionization energy that electrons with more full valence shells tend to attract electrons, and elements with less full shells tend to give away electrons.
In this sense, electronegativity is the opposite of ionization energy, trend-wise, because the electronegativity is higher if the valance shell is more full, and lower if it is less full.
Going top to bottom in a group, the electronegativity generally decreases.
This due to the atomic radius of the atom: the farther down a group, the larger its radius (discussed above) and the farther the outer electrons are from the nucleus. With a larger radius, the less of a pull the nucleus has on the electrons, so the likelihood of bonding electrons to drift toward the larger atom is less.
Here is a periodic table showing electronegativity values on the Pauling scale:
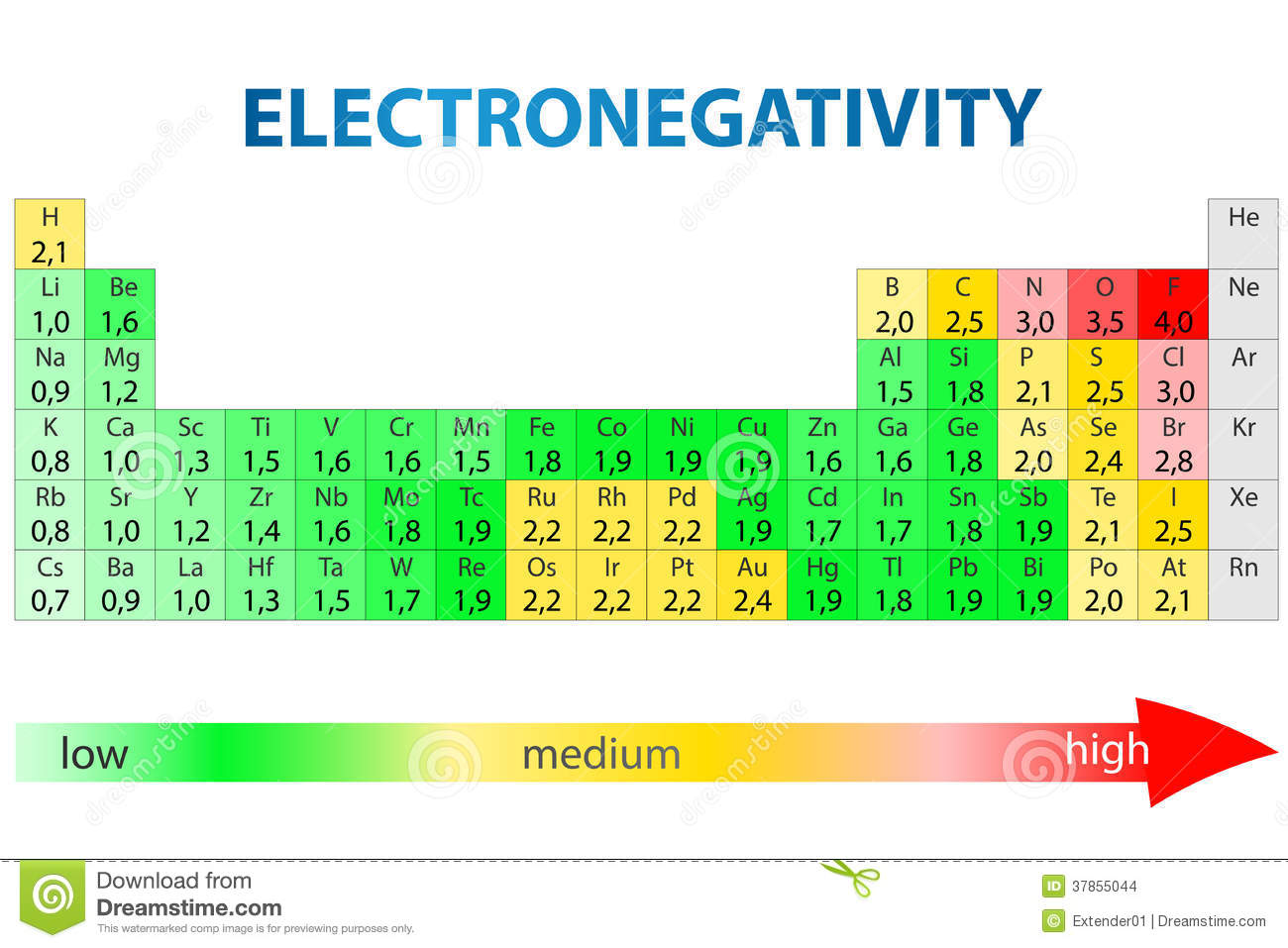
The electron affinity of an element is the energy change associated with the addition of an electron; i.e. it is also a measure of the ability of an atom to attract electrons to itself.
Therefore, it will follow nearly the exact same trends as those for electronegativity: increases left to right and decrease top to bottom.
The metallic character of an element is typically defined as how much the atom behaves like metal in the sense that they tend to lose electrons to form cations.
The metallic character tends to decrease when going from left to right across a period.
For reasons as described above, when going left to right the energy needed to remove an electron increases, so it is less likely to remove an electron and form a cation.
Metallic character tends to increase going down a group.
For reasons as described above, elements toward the bottom of a group are farther from the nucleus and require less energy to remove the electron, so it more easily loses an electron and forms a cation.
Here is a neat periodic table illustrating the trends above:
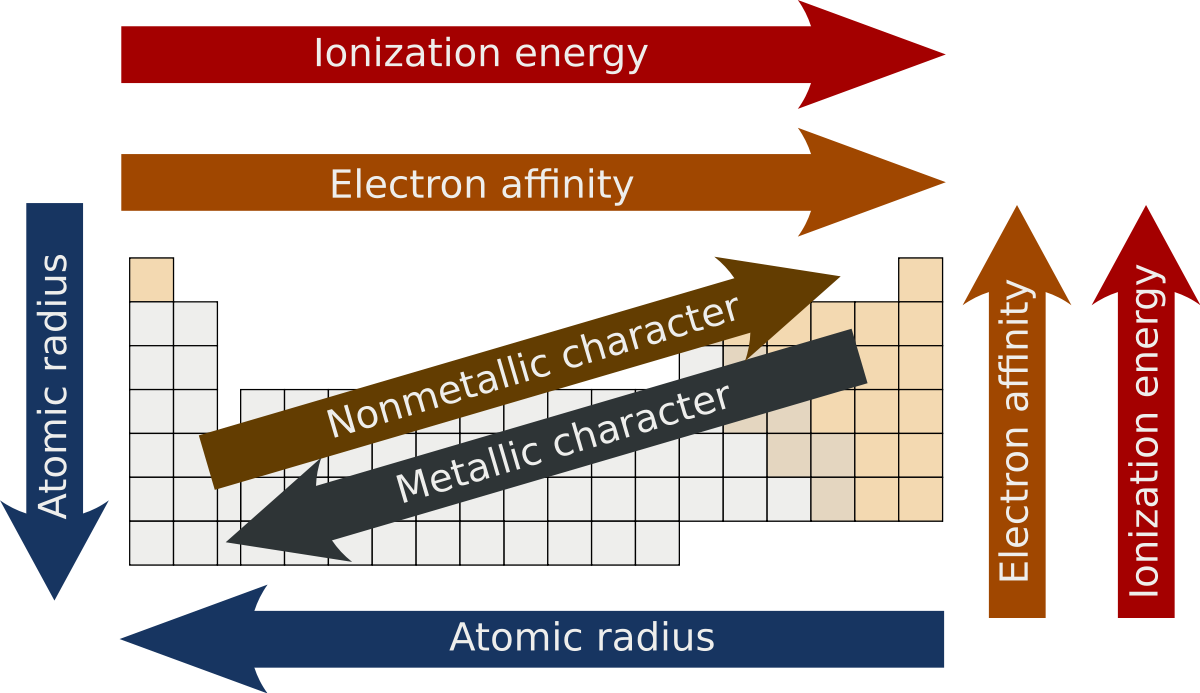
(Just know here that electronegativity and electron affinity follow the same trend.)
