Question #7259d
1 Answer
Jun 21, 2017
- There is a buffer region in the strong base / weak acid titration curve where the
#"pH"# rises slowly, but not the strong base / strong acid titration. - For the strong base / weak acid titration curve, we do have a half-equivalence point where
#"pH"# #~~# #"pKa"# , but that relationship does not hold true for the strong base / strong acid titration curve. #"pH"# at the equivalence point is greater than#7# for the strong base / weak acid titration and equal to#7# for the strong base / strong acid titration.
The main qualities (without identified differences yet) you should expect from each kind of titration curve are:
Both curves
- Starting
#"pH"# of what is being titrated (what the titrant from the burette is being dripped into) is less than#7# (after the equivalence point). - End
#"pH"# of what is being titrated is greater than#7# (after the equivalence point).
Strong base into strong acid
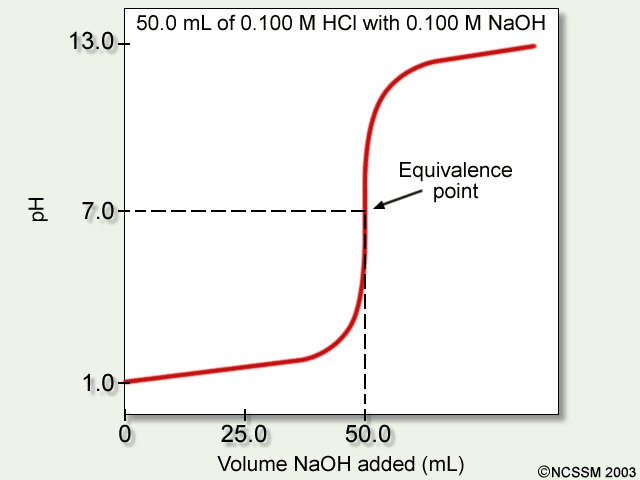
- There is no buffer region on the way to the equivalence point, because we need a weak acid / conjugate base or weak base / conjugate acid combination to have this. This lack of a buffer region is seen as a simple flat rise.
- There is a half-equivalence point, but the
#"pH"# is simply given by the concentration of#"H"^(+)# in the solution, and bears no relationship to the#"pKa"# of the acid. #"pH"# at the equivalence point is exactly#7# (exact neutralization of all#"H"^(+)# and#"OH"^(-)# that are not from water).
Strong base into weak acid
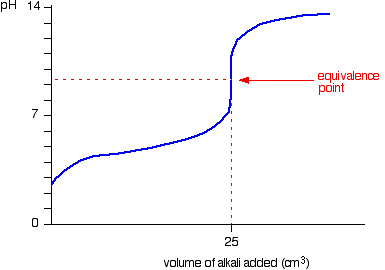
- There is a buffer region on the way to the equivalence point where the
#"pH"# increases slowly. This is seen as an initial "hill" into a flat rise. - There is a half-equivalence point, i.e. half the volume of the equivalence point, which has
#"pH"# #~~# #"pKa"# (from the Henderson-Hasselbalch equation), where#"pKa" = -logK_a# and#K_a# is the acid dissociation constant. #"pH"# at the equivalence point is greater than#7# . You can think of it as the strong base "dominating" the resultant#"pH"# at that point.
DIFFERENCES
In the end, we can summarize the following differences:
- There is a buffer region in the strong base / weak acid titration curve where the
#"pH"# rises slowly, but not the strong base / strong acid titration. - For the strong base / weak acid titration curve, we do have a half-equivalence point where
#"pH"# #~~# #"pKa"# , but that relationship does not hold true for the strong base / strong acid titration curve. #"pH"# at the equivalence point is greater than#7# for the strong base / weak acid titration and equal to#7# for the strong base / strong acid titration.

