The second ionization energy (#"IE"_2#) is the energy required to remove an electron from a 1+ cation in the gaseous state.
#"X"^"+""(g)" → "X"^"2-""(g)" + "e"^"-"#
Just like the first ionization energy, #"IE"_2# is affected by size, effective nuclear charge, and electron configuration.
We would expect second ionization energies to increase from left to right as the ionic size decreases.
Here's a table listing the electron configurations of the ions involved.
#bb("Group"color(white)(m)"Element"color(white)(m)"Config"color(white)(m)"Ion"color(white)(m)"Config")#
#color(white)(mll)1 color(white)(mmmmll)"Li"color(white)(mmml)"2s"color(white)(mmml)"Li"^"+"color(white)(mm)"1s"^2"#
#color(white)(mll)2color(white)(mmmmll)"Be"color(white)(mmm)"2s"^2color(white)(mmm)"Be"^"+"color(white)(mll)"2s"#
#color(white)(m)13color(white)(mmmmm)"B"color(white)(mmml)"2s"^2 "2p"color(white)(mll)"B"^"+"color(white)(mml)"2s"^2#
#color(white)(m)14color(white)(mmmmm)"C"color(white)(mmml)"2s"^2 "2p"^2color(white)(ml)"C"^"+"color(white)(mml)"2s"^2 "2p#
#color(white)(m)15color(white)(mmmmm)"N"color(white)(mmml)"2s"^2 "2p"^3color(white)(ml)"N"^"+"color(white)(mml)"2s"^2 "2p"^2#
#color(white)(m)16color(white)(mmmmm)"O"color(white)(mmml)"2s"^2 "2p"^4color(white)(ml)"O"^"+"color(white)(mml)"2s"^2 "2p"^3#
#color(white)(m)17color(white)(mmmmm)"F"color(white)(mmmll)"2s"^2 "2p"^5color(white)(ml)"F"^"+"color(white)(mml)"2s"^2 "2p"^4#
#color(white)(m)18color(white)(mmmmm)"Ne"color(white)(mmm)"2s"^2 "2p"^6color(white)(ml)"Ne"^"+"color(white)(mll)"2s"^2 "2p"^5#
And here is a plot of the ionization energies.
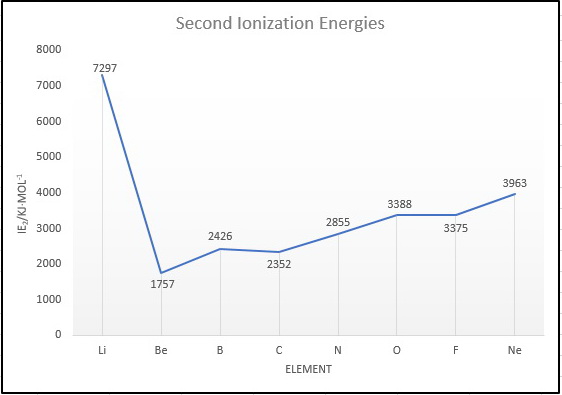
We notice three things:
-
#"Li"# has the highest #"IE"_2#, because to remove the second electron we must break the stable #"1s"^2# noble gas shell.
-
#"B"# has a greater #"IE"_2# than #"C"#. This is probably due to the extra stability of the #"s"^2# subshell in the #"B"^"+"# ion.
-
#"O"# has a greater #"IE"_2# than #"F"#. The #"F"^"+"# ion has a #"p"^4# configuration in which electronic repulsions raise the energy and decrease the #"IE"_2#.
Thus, the order of second ionization energies is
#"Be < C < B < N < F < O < Ne < Li"#.


