What temperature range are most elements on the periodic table liquids and how many would be liquids?
3 Answers
Explanation:
To find the temperature at which the largest number of elements will be liquid, you need the element with the highest melting point and its melting point.
Doesn't melt under normal conditions
And that is carbon(diamond highest melting point of all allotropes) with a melting point of
This is only for the known chemical elements.
As it has covalent bonds, it is much stronger than the metallic bond, the strength relates itself with the atomic vibration (temperature) needed for a disassembling of the long-range structure and therefore the formation of a liquid phase.
Covalent bonds are as strong as ionic bonds which are not possible in other elements because their molecules form network covalent structures. These structures form a lattice-like structure, much the same as ionic compounds.
The network structure combines to make the substance stronger than normally covalently bonded substances and sometimes even stronger than ionic bonds which is the present case.
In single covalent bonds you have the overlapping of orbitals but in this you have many (4) atoms joined to "one" atom covalently. The structure becomes stronger when there are more atoms joined to one atom covalently. Like graphite has 3 carbon atoms joined to one atom thus it has a lower melting point.
Under normal conditions
But if you want to know the element with the highest melting point under normal conditions it is tungsten which has a melting point of 3414 °C But then the other elements can be gas, so it is estimated of around
You haven't specified the pressure, so I will assume
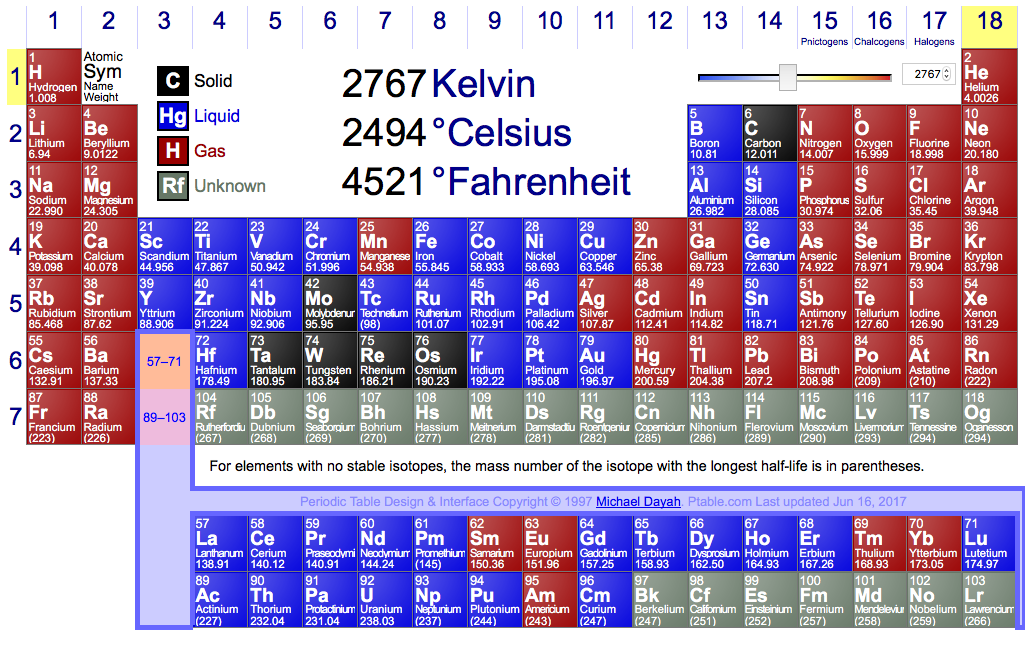
Here, elements highlighted blue are liquids at this
Of course, I may have missed a better temperature range, but this is a tall order for a question...
Around 2700 K at a pressure of 100,000 Pa with 42 elements in their liquid state.
Explanation:
Vanadium melts at just under 2200 K and Aluminium boils at just under 2800 K, any value between that has 42 elements in their liquid states.
At 2767 I had 42 elements at liquid, though there might be a better temperature.