Question #aa923
1 Answer
The number of orbitals present in a given subshell.
Explanation:
For starters, you should know that the angular momentum quantum number,
The magnetic quantum number,
As you know, orbitals are located in subshells, which in turn are located in energy shells.
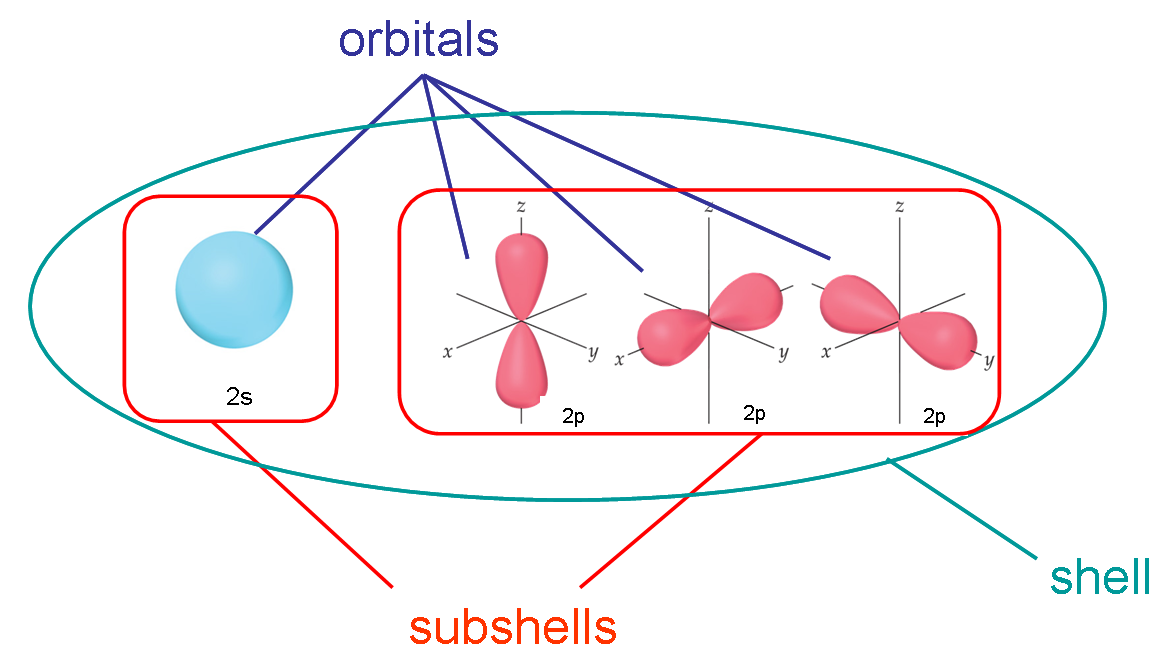
This implies that the values that the magnetic quantum number can take depend on the values of the angular momentum quantum number, which, of course, depend on the values of the principal quantum number,
Now, the number of orbitals located in a given subshell is given by
#color(blue)(ul(color(black)("no. of orbitals" = 2l + 1)))#
In other words, the number of values that the magnetic quantum number can take for a given subshell, i.e. for a given value of
Let's take the example illustrated in the image.
For the second energy level,
#l = 0 -># denotes the#2s# subshell#l=1 -># denotes the#2p# subshell
Now, the
#"no. of orbitals in 2s" = 2 * 0 + 1 = 1#
The
#"no. of orbitals in 2p" = 2 * 1 + 1 = 3#
In other words, the magnetic quantum number can only take
#n=2, l=0 implies m_l = 0#
and
#n=2, l=1 implies {(m_l = -1), (m_l = 0), (m_l = +1) :}#
You now know that

