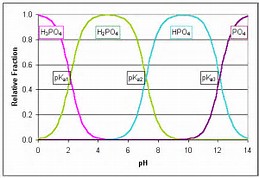
With respect to acidity, how does #H_3PO_4# behave in aqueous solution?
1 Answer
Jul 1, 2017
Well, phosphoric acid is a fairly WEAK acid.......but in aqueous solution it is a diacid.........
Explanation:
The third dissociation is largely irrelevant and non-operative in aqueous solution........You will have to look up