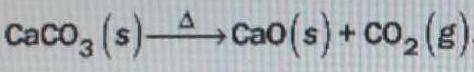
Calcium oxide, or lime, is produced by the thermal decomposition of limestone in the following reaction. What mass of lime can be produced from #1.5 x 10^3# kg of limestone?
1 Answer
Approx.
Explanation:
You have the stoichiometric equation, which tells you UNEQUIVOCALLY that
If you haven't already realized
With respect to
And thus if such a molar quantity of calcium carbonate were reacted we would get a molar quantity of
We also get
In what industry do you think that these reactions would have direct relevance? Or rather in what industry are these reactions routinely performed on these scales or larger?

