How can you tell which atoms are in the same plane of cumulene? Why is allene preferentially twisted instead of planar?
1 Answer
Well, you kinda just have to know when it comes to these two types of compounds...
Cumulene is an entirely planar molecular class, but allene is more stable as a twisted conformer. As a note, a cumulene can have three OR MORE consecutive double bonds.
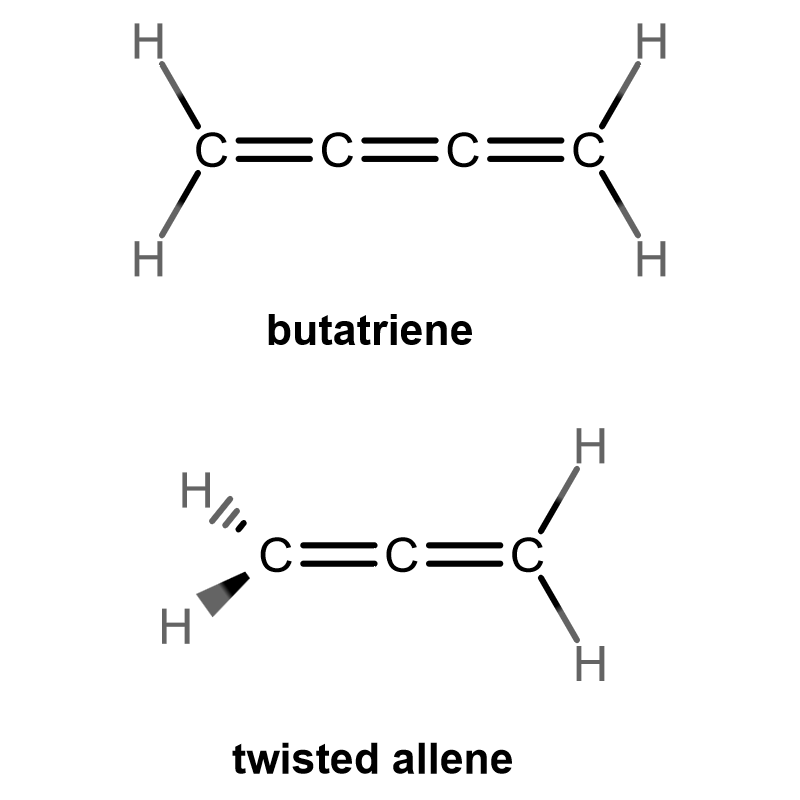
Here, butatriene has all atoms in the same plane, and in principle, a cumulene is entirely planar. Allene has all planar atoms EXCEPT the left-hand hydrogens.
Even though the entire carbon chain consists of double bonds on both molecules, half of allene is
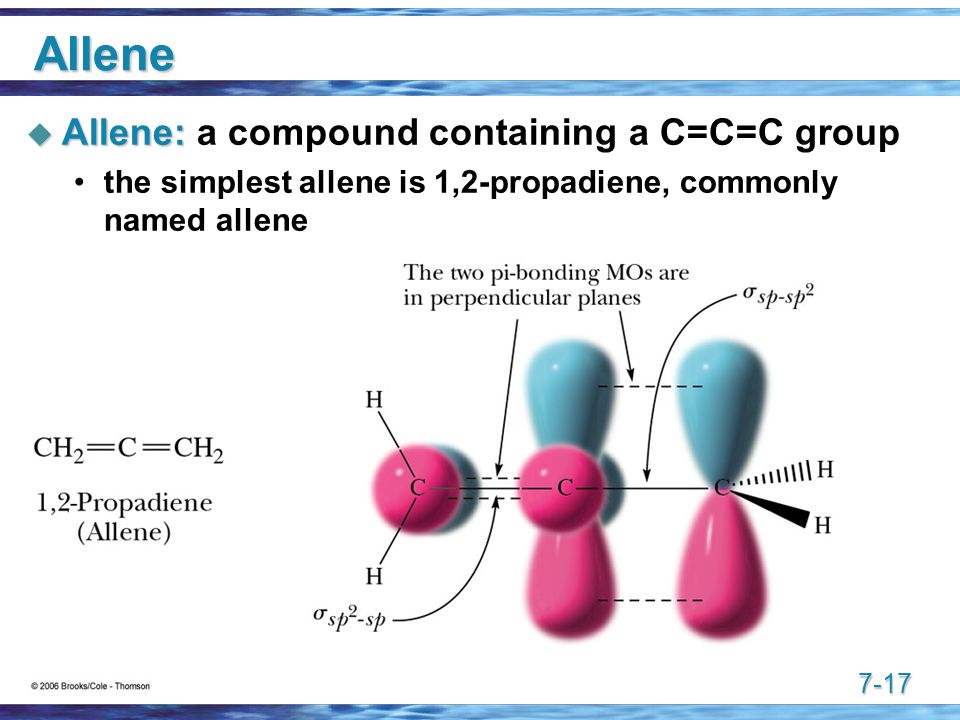
I would think that allene is twisted so that the same

