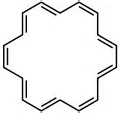
How many #pi-"electrons"# in benzene?
1 Answer
Jul 9, 2017
How many double bonds do you see?
Explanation:
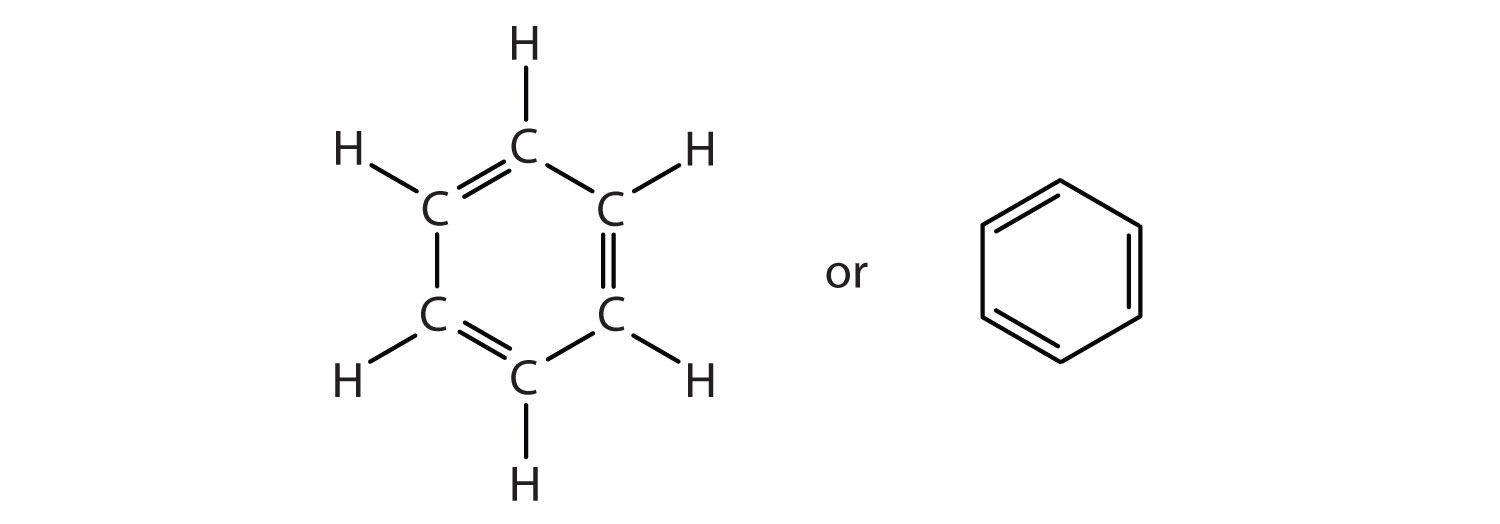
I see 3 here..........And each such double bond represents
And since there are 3 bonds, there are
The alternative representation of benzene, an hexagon circumscribing a circle.......
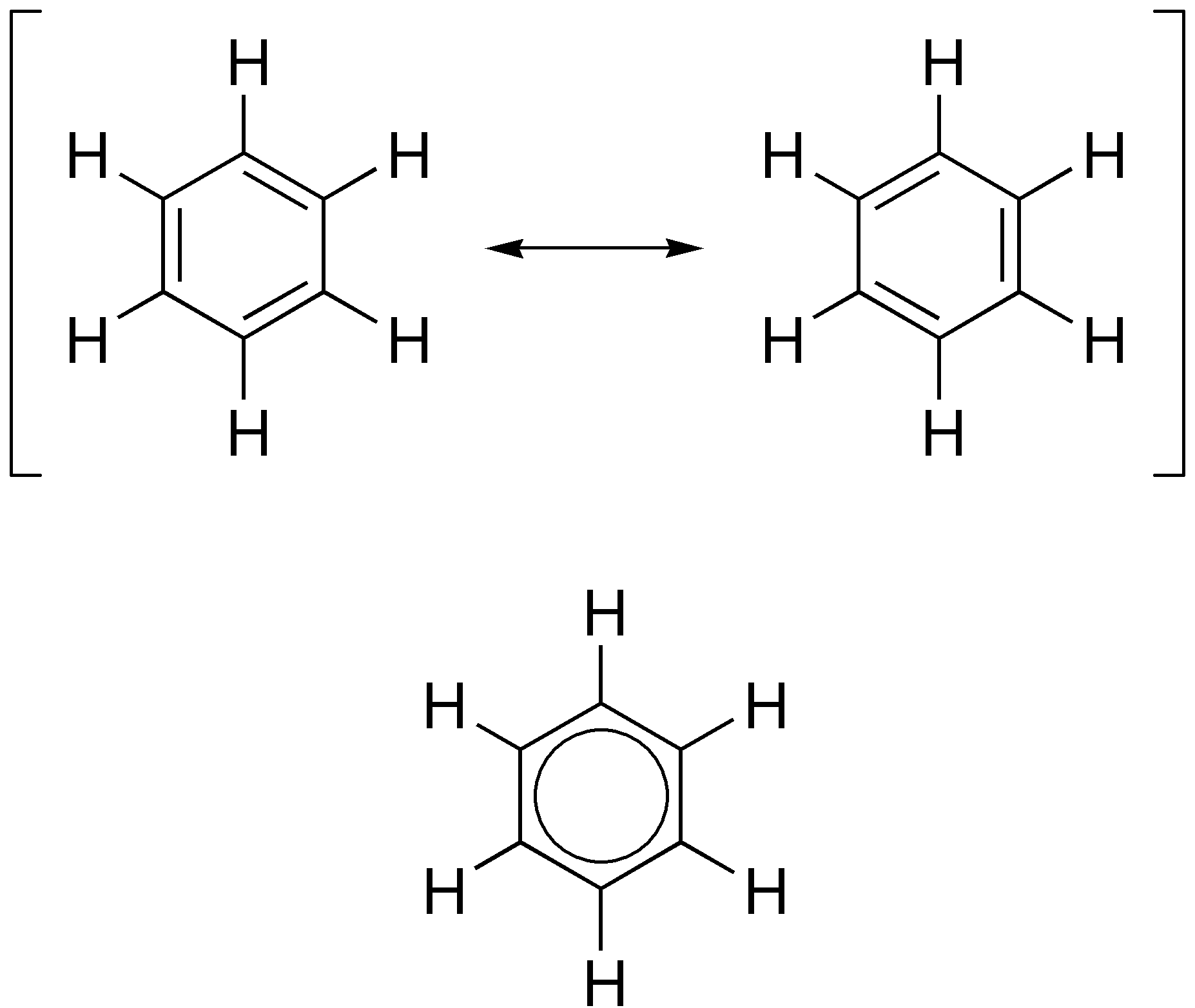
............must be understood to represent those same
How many in this system........?