In spite of having positive charge on the ions # H_3O^+# as well as #NH_4^+#, why are they not considered as electrophiles?
1 Answer
Jul 10, 2017
Because they cannot directly bond to a nucleophile, due to having no vacant orbitals.
An electrophile must be able to directly bond to a nucleophile by accepting an electron pair.
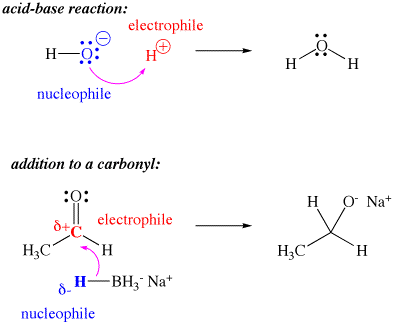
In

