How does "electron affinity" change across the Periodic Table?
1 Answer
Jul 10, 2017
If we discount the Noble Gases, then electron affinity should INCREASE across the Period from left to right as we face the Table....
Explanation:
We assess the thermodynamics of the reaction........
And
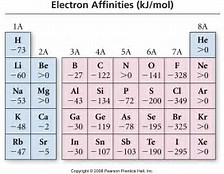 imgarcade,com
imgarcade,com
Does this data set support my argument? Why or why not?

