What kind of bonding is responsible for the slight solubility of air in water?
1 Answer
Jul 13, 2017
There is no bonding involved here.
Air dissolves in water by interspersing itself amongst water molecules. Air consists entirely of gas molecules and atoms, which tend to be only slightly soluble in water (particularly if the molecules aren't polar, but nonpolar instead).
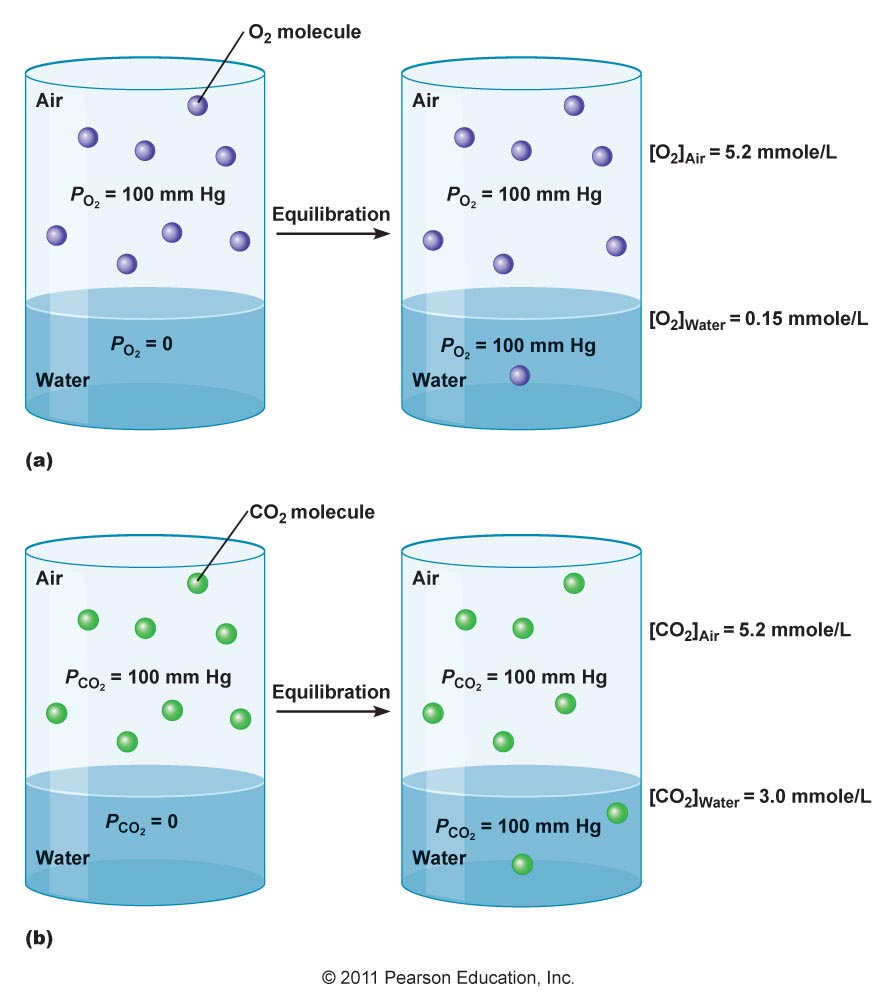
The slight solubility is also in part due to gases simply being fast-moving particles, having a high escaping tendency (or activity coefficient).
The main intermolecular force that allows this slight solubility is the weakest of them all, London Dispersion, since air is mostly
I reiterate: no chemical bonding is involved.

