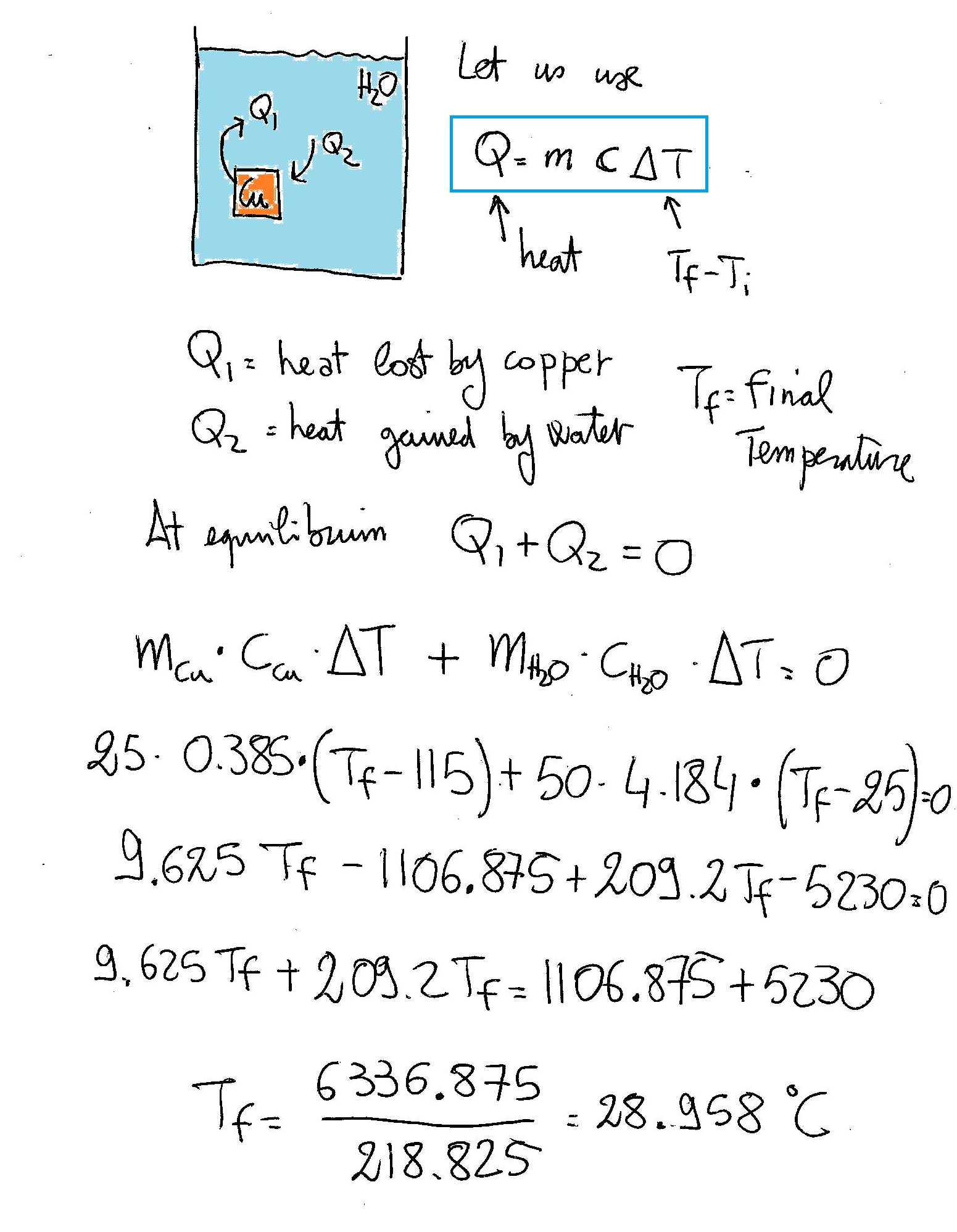
If a 25.00g piece of copper (C_s = (0.385J)/(g*°C)) at 115.0°C is added to 50.00g of water (C_s = (4.184J)/(g*°C)) at 25.00°C, what is the final temperature once the object and water thermally equilibrate?
1 Answer
Jul 14, 2017
I got almost
Explanation:
Have a look: