Whats the difference between ez isomerism and cis and trans isomers? (they both seem to basically have the same rules so i don't get the difference)
1 Answer
Jul 14, 2017
They are the same,
Take the two isomers for 1,2-dichloroethene for example:
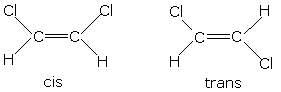
As each side only has 1 H and 1 Cl atom, you can tell if the Cl atoms are on the same or opposite sides, and therefore decide upon cis- or trans-.
However, if you took:

Each 'corner' has something different, there is nothing to compare each other too. However, EZ notation uses the total atomic mass of each corner to decide upon E- or Z-. In this case, on the left hand side

