Question #fb349
1 Answer
Here's what I got.
Explanation:
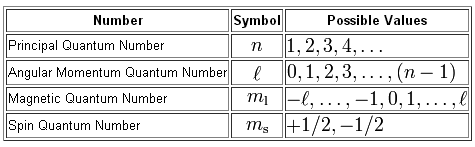
As you know, we can use four quantum numbers to describe the position and spin of an electron in an atom.
I assume that you're fairly familiar with how they work, so I won't go into too much detail here.
So, you know that you must find the sets of quantum numbers that can be used to describe an electron located in the
The number added in front of the subshell tells you the energy level on which the subshell is located, i.e. the value of the principal quantum number,
In your case, you have the
#n = 4 -># the fourth energy level
The
#l = 2 -># the d subshell
For the
#m_l = {-2, -1, 0, 1, 2} -># five orbitals in the d subshell
Each value of the magnetic quantum number describes one of the five orbitals present in the
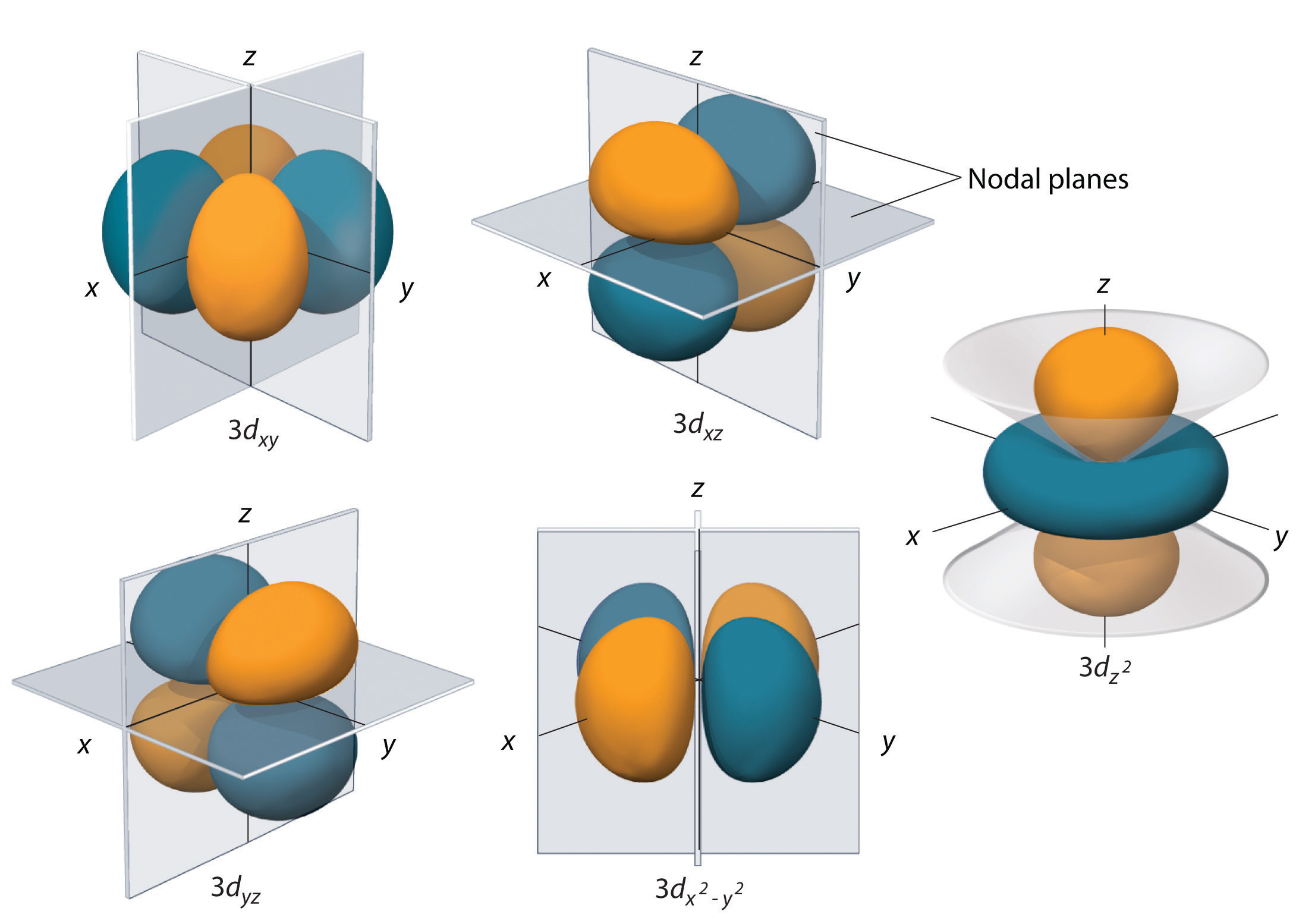
Finally, each orbital can hold a maximum of
#m_s = {-1/2, 1/2} -># two electrons of opposite spins
So, you can say that the
#5 color(red)(cancel(color(black)("orbitals"))) * ("2 e"^(-))/(1color(red)(cancel(color(black)("orbital")))) = "10 e"^(-)#
that can be described using the following sets of quantum numbers--each individual orbital is shown in a different color
#n=4, l=2, color(blue)(m_l = -2), m_s = -1/2# #n=4, l=2, color(blue)(m_l = -2), m_s = +1/2# #n=4, l=2, color(purple)(m_l = -1), m_s = -1/2# #n=4, l=2, color(purple)(m_l = -1), m_s = +1/2# #n=4, l=2, color(red)(m_l = 0), m_s = -1/2# #n=4, l=2, color(red)(m_l = 0), m_s = +1/2# #n=4, l=2, color(darkgreen)(m_l = 1), m_s = -1/2# #n=4, l=2, color(darkgreen)(m_l = 1), m_s = +1/2# #n=4, l=2, color(darkorange)(m_l = 2), m_s = -1/2# #n=4, l=2, color(darkorange)(m_l = 2), m_s = +1/2#

