At what absolute temperatures do molecules occupy each energy level?
1 Answer
At absolute what temperature? Absolute temperature is in units of
- All molecules have translational energy at most temperatures, except
#"0 K"# . - All molecules have ONLY vibrational energy at
#"0 K"# . - As you increase the temperature from
#"0 K"# , molecules begin to gain rotational energy (usually before#"100 K"# ), and at really high temperatures (a few thousand#"K"# ), they gain vibrational energy. - At really, really, really high temperatures (tens of thousands of
#"K"# , probably), they gain electronic energy.
DISCLAIMER: LONG ANSWER!
The most common different kinds of energies are:
- translational
- rotational
- vibrational
- electronic
These energy levels are in general differently spaced as follows:
#"electronic"# #">>"# #"vibrational"# #># #"rotational"# #">>"# #"translational"#
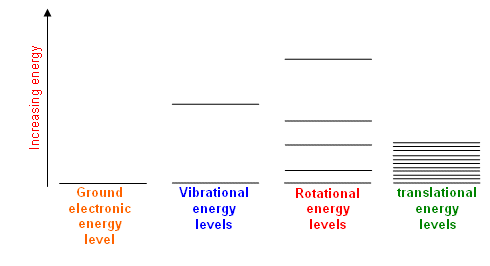
For the temperatures that we care about, these temperatures are called the rotational and vibrational temperatures,
We have:
#Theta_(rot) = (B_e)/(k_B)# in units of#"K"# , usually in the tens of#"K"#
#Theta_(vib) = (omega_e)/(k_B)# in units of#"K"# , usually in the hundreds or thousands of#"K"#
#Theta_(el ec) = (T_e)/(k_B)# in units of#"K"# , usually in the upper thousands or tens of thousands of#"K"# where:
#B_e# is the rotational constant in#"cm"^(-1)# for each type of rotational motion (up to#2# for linear molecules and#3# for nonlinear molecules).#omega_e# is the fundamental vibrational frequency in#"cm"^(-1)# for each vibration (the number of vibrational motions are#3N - 5# for linear polyatomic molecules and#3N - 6# for nonlinear polyatomic molecules, where#N# is the number of atoms).#T_e# is the electronic frequency constant (or whatever it's called), and is analogous.#k_B ~~ "0.695 cm"^(-1)"/K"# is the Boltzmann constant in#"cm"^(-1)"/K"# .
And when these temperatures are surpassed, the molecule has that kind of energy.
In summary...
- All molecules have translational energy at most temperatures, except
#"0 K"# . - All molecules have nonzero vibrational energy at
#"0 K"# . - As you increase the temperature from
#"0 K"# , molecules begin to gain rotational energy (usually before#"100 K"# ), and at really high temperatures (a few thousand#"K"# ), they gain vibrational energy. - At really, really, really high temperatures (tens of thousands of
#"K"# , probably), they gain electronic energy.
EXAMPLE MOLECULE: CO
As an example (http://webbook.nist.gov/cgi/cbook.cgi?ID=C630080&Units=SI&Mask=1000):
#"CO"# has a rotational frequency at around#"1.93 cm"^(-1)# , so only past around
#"1.93 cm"^(-1)/("0.695 cm"^(-1)"/K") ~~ ul"2.78 K"# would it rotate from natural surrounding heat.
#"CO"# has a vibrational frequency at around#"2169.8 cm"^(-1)# , so only past around
#"2169.8 cm"^(-1)/("0.695 cm"^(-1)"/K") ~~ ul"3122 K"# would it vibrate from natural surrounding heat.
#"CO"# has its first excited-state electronic energy level about#"48686.7 cm"^(-1)# above the ground state, which means it won't be until at least
#"48686.7 cm"^(-1)/("0.695 cm"^(-1)"/K") ~~ ul"70000 K"# or so before
#"CO"# has electronic energy naturally from surrounding heat.

