Question #138dd
1 Answer
Well, I'll address a core concept found in questions
#"Ag"_2"O"(aq) + "Zn"(s) -> "2"Ag"(s) + "ZnO"(aq)#
The battery is also a galvanic cell, where zinc is oxidized from
#(a)# is not correct, because#"Ag"_2"O"# is a reactant... reactants get consumed, and you should know this from the definition of a reactant.#(b)# is not correct, for two reasons.#"Ag"_2"O"# is aqueous, and thus is not an anode, a solid. Furthermore,#"Zn"# gets oxidized from#stackrel(0)"Zn"# to#stackrel(+2)"Zn"# in#"ZnO"# .
Hence,
#"Zn"# is the anode, OUT of which electrons flow to get TO the positive terminal and reduce the aqueous#"Ag"_2"O"# to increase#"Ag"(s)# mass at the cathode.
#(c)# We just said the opposite... Electrons flow AWAY from zinc, so#"Zn"# gets oxidized, not reduced. Thus, this is incorrect.#bb((d))# is thus correct, as the anode is the source of the reducing electrons.
Besides that...
You must be able to read a standard reduction potential table, not unlike this one:
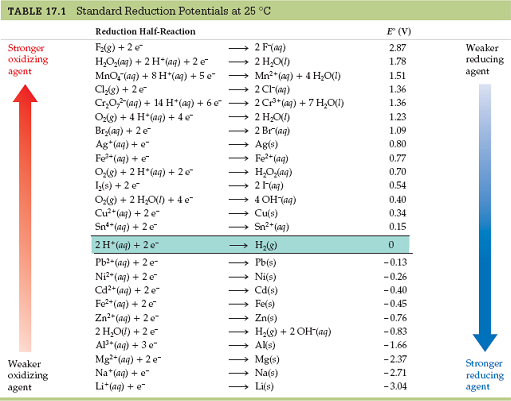
And it is mandatory that you receive a table like this at any chemistry exam where these questions are asked. Or, you should be able to figure out where it is in your textbook.
Either of these conclusions can be made by looking at the left and right sides of the table:
- Any reactant that is higher up on the standard reduction potential table is more easily reduced and thus a better oxidizing agent.
- Any reactant that is lower in the standard reduction potential table is more easily oxidized and thus a better reducing agent.
And some general rules:
- A cation usually won't get further oxidized (with one exception being
#"Fe"^(2+) -> "Fe"^(3+)# , among others). - A polyatomic oxygen-based ion (
#"MnO"_4^(-)# ,#"CrO"_4^(2-)# , etc.) probably won't be oxidized, but would rather be an oxidizing agent. - A reduction reaction from the table above, when reversed, will reverse the sign of
#E^@# for the half-reaction. - A spontaneous reaction has
#E_(cell)^@ > 0# , where#E_(cell)^@ = E_(red)^@ + E_(o x)^@# .

