Question #638eb
1 Answer
due to presence of empty orbitals
Explanation:
The answer to this question is not provided by the bohr atomic model.
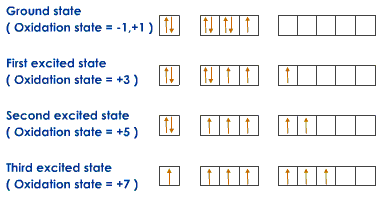
According to the quantum mechanical model electrons do not revolve around the nucleus in fixed orbits.Instead they move in orbitals which are areas around the nucleus where the probability of finding a electron is more than 80%.There are shells,subshells and orbitals.Subshells are present in the shells which have a definite number of orbitals .The shape of these orbitals depends on the subshells.
Thus the 3rd shell for example contains three subshells:s,p,d.The s subshell has 1 orbital, p has 3, and d has 5.To form a bond, an element must have unpaired electrons.Now if an element say chlorine has to form 7 bonds,it transports some of its electrons into the d subshell.Thus it has variable valancy.