Question #65110
1 Answer
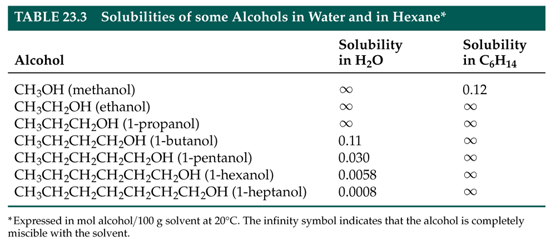
(i) the presence of functional groups that promote water solubility....
Explanation:
(i) the presence of functional groups, for instance, alcohol, carboxyl, carbonyl, or amino, all of which would promote water solubility....
and (ii) the absence of an exceptionally large hydrocarbyl chain.
So were we to consider the water solubility or
 answers.yahoo.com
answers.yahoo.com
Of course, we have only dealt with alcoholic solubility. Carboxylic acids,
Are you happy with this?

