Well, if you insert the #f# block elements in their proper place, you would have an extended periodic table:
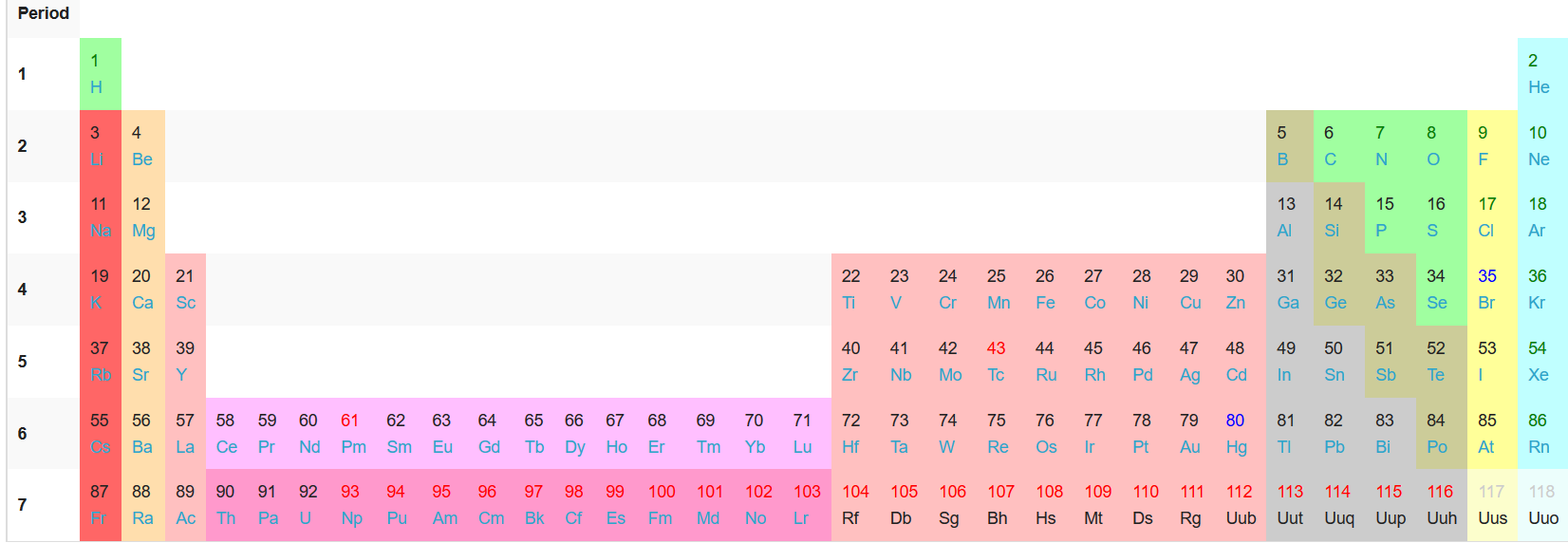
And so, the #f# block elements are "inner" transition elements, being between the #"Sc"# group and the rest of the #d# block. The #d# block is then "outer", as they are on either side of the "inner" transition metals.
The #f# block elements have rather unusual electron configurations, and you probably won't need to know them in your lifetime. Should you ever want to just for fun, I've written a detailed explanation of why there are so many exceptions to their electron configurations.

